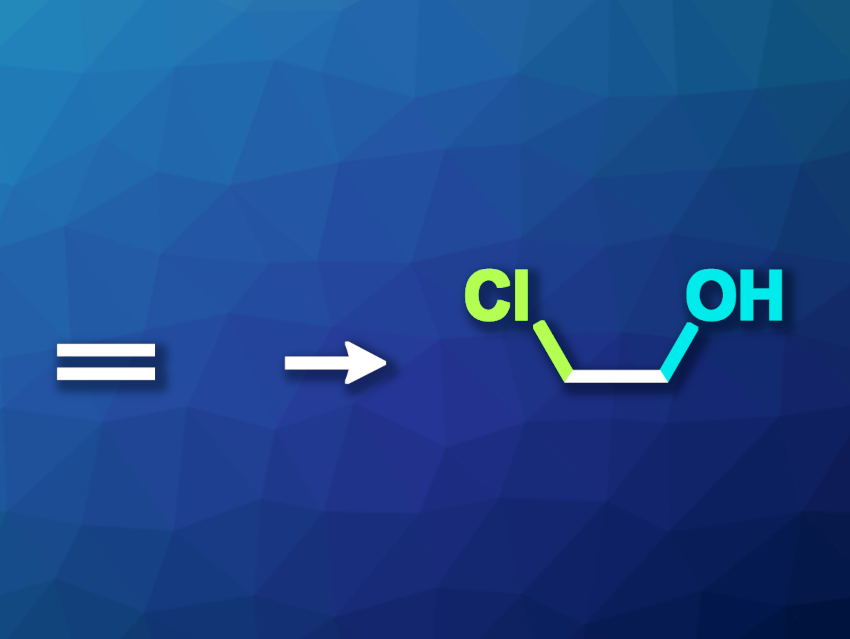
The oxidation of ethylene can be used to prepare useful products such as ethylene glycol, acetaldehyde, or ethylene oxide. Usually, this type of transformation requires high temperatures and pressures. Electrooxidation can provide a more environmentally friendly alternative. However, so far this approach has been limited to alkaline and neutral electrolytes, giving acetaldehyde and ethylene glycol.
Yao Zheng, Shi-Zhang Qiao, The University of Adelaide, Australia, and colleagues have performed ethylene electrooxidation over a Pd-based catalyst in the presence of chloride to convert ethylene to 2-chloroethanol. The team used seawater as a chloride-containing electrolyte for this reaction and performed it under strongly acidic conditions. They incorporated seawater collected from the Australian shore in a typical three-electrode flow cell or a proton exchange membrane (PEM) electrolyzer and adjusted the pH value using HClO4. The PEM electrolyzer used Pt/C and Pd/C as the cathode and anode, respectively.
The team found that the Faradaic efficiency for 2-chloroethanol production in acidic seawater reached ca. 68 % at 2.2 V. They propose a mechanism for the formation of 2-chloroethanol at low potentials that involves the direct interaction of adsorbed chloride anions with ethylene instead of a conventional Cl2-mediated reaction at high potentials.
- Ethylene Electrooxidation to 2-Chloroethanol in Acidic Seawater with Natural Chloride Participation,
Linsen Huang, Pengtang Wang, Yunling Jiang, Kenneth Davey, Yao Zheng, Shi-Zhang Qiao,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c05114