Safe and efficient energy storage under high-temperature conditions can be challenging to achieve and requires advanced battery technologies. At this stage, widely used lithium-ion batteries are not entirely safe because they contain flammable organic electrolytes. Zinc–air batteries (ZABs) could have potential as next-generation battery systems due to their high theoretical energy density, environmental friendliness, and low cost. ZABs use non-flammable aqueous electrolytes, which are inherently safer in higher temperatures.
Qiang Zhang, Tsinghua University, Beijing, China, Bo-Quan Li, Beijing Institute of Technology, China, and colleagues have systemically investigated the operation of aqueous zinc–air batteries (ZABs) at high temperatures. They examined temperature effects on the electrolyte, cathode, and anode.
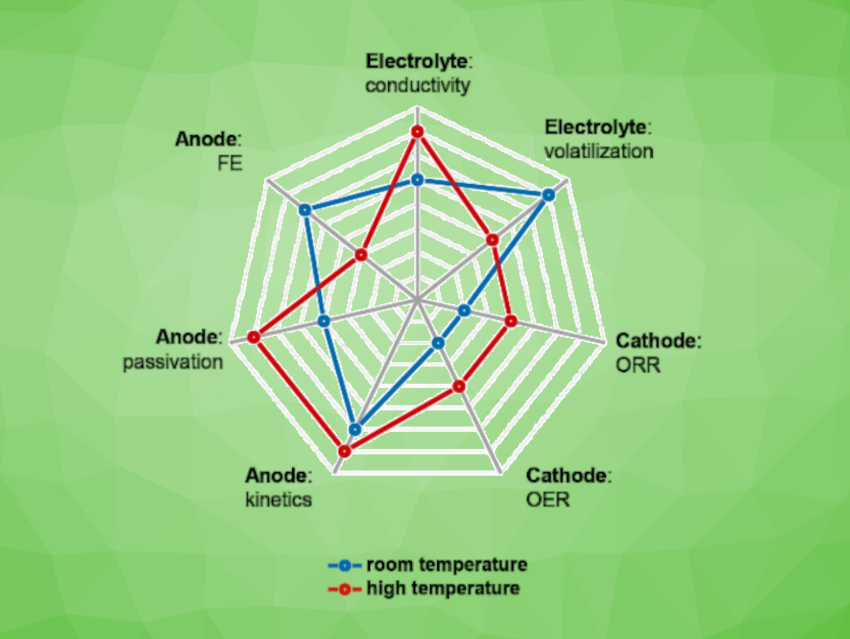
The team found that the main bottleneck at high temperatures is a deterioration in the Faraday efficiency (FE) of the anode, stemming from a more prominent parasitic hydrogen evolution reaction (HER). The HER competes with zinc deposition and the FE is only 56.7 % at 80 °C and a current density of 25 mA cm−2. In contrast, the electrolyte conductivity, the electrode kinetics, and the effects of anode passivation were improved at higher temperatures.
Nevertheless, the researchers showed that zinc–air batteries can work at higher temperatures: A ZAB containing an electrolyte with 6 M potassium hydroxide and 0.2 M zinc acetate with a boiling point of 115 °C was successfully cycled at 80 °C. This indicates that a reduced FE and the increased volatilization of the electrolyte are not necessarily “deadly” to ZABs. The researchers believe that ZABs could be operational under a wider range of working conditions than commonly thought.
- Working Zinc–Air Batteries at 80 °C,
Chang-Xin Zhao, Legeng Yu, Jia-Ning Liu, Juan Wang, Nan Yao, Xi-Yao Li, Xiang Chen, Bo-Quan Li, Qiang Zhang,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202208042