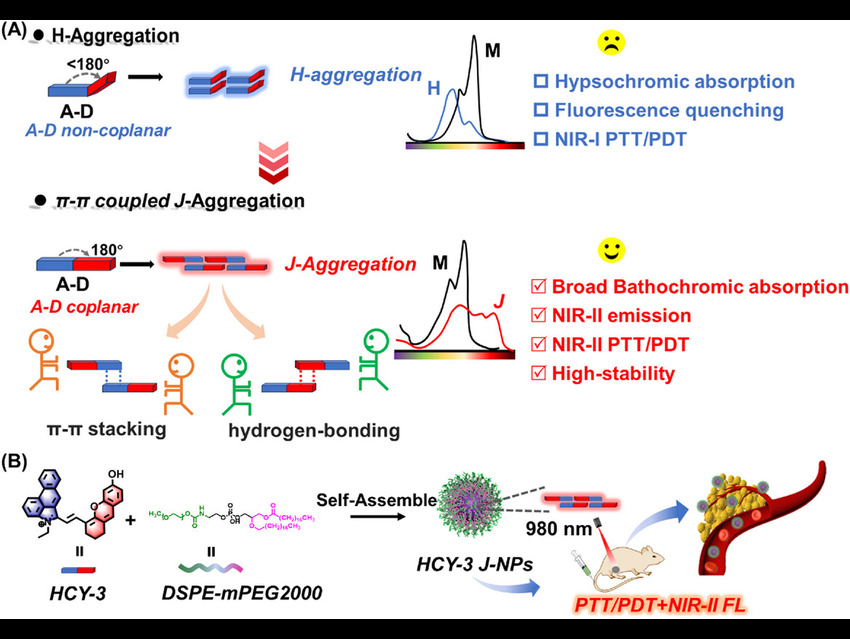
Conventional photosensitizers often suffer from poor stability and limited absorption in the near-infrared (NIR) region, which reduces their effectiveness in phototherapy. Yincheng Chang and Fang Sun, Beijing University of Chemical Technology, China, and colleagues aimed to overcome these limitations by designing a photosensitizer that remains stable under heat and light while extending absorption into the NIR-II window (1000–1700 nm).
HCY-3 was designed as a hemicyanine dye (HCY) with hydroxyl donor groups to promote hydrogen bonding. Comparisons with structural variants (S-HCY-1, HCY-4, and HCY-9) confirmed that molecular planarity and donor–acceptor alignment are critical for J-aggregation (the ordered head-to-tail stacking of dye molecules that red-shifts absorption and enhances stability and emission).
Spectroscopic studies showed that HCY-3 forms stable J-aggregates in protic solvents, exhibiting broad bathochromic absorption and remarkable thermal stability even at 100 °C, unlike the reversible aggregation observed for HCY-2. Density functional theory calculations indicated that HCY-3 has a smaller HOMO–LUMO gap, stronger intramolecular charge transfer, and enhanced intersystem crossing, accounting for its red-shifted absorption and efficient singlet oxygen generation. Molecular dynamics simulations further validated the J-aggregate geometry, identifying antiparallel stacking with an interlayer spacing of 3.782 Å and a slip angle of 22.66°.
To improve solubility and biocompatibility, HCY-3 was encapsulated in the amphiphilic polymer DSPE-mPEG2000 to form nanoparticles (~85 nm). These nanoparticles showed strong NIR absorption at 960 nm, NIR-II fluorescence emission at 925 nm, and a photothermal conversion efficiency of 40.2%. Biological evaluation confirmed that the nanoparticles generate reactive oxygen species and heat under 980 nm laser excitation, enabling effective photodynamic and photothermal therapy (PDT and PTT). In vivo imaging showed selective tumor accumulation via the enhanced permeability and retention effect, peaking at 12 h post-injection. Subsequent laser treatment raised tumor temperatures to 62.2 °C, causing apoptosis and necrosis (programmed and uncontrolled cell death) without toxicity to major organs.
Collectively, the researchers identified a new class of π–π-coupled J-aggregates combining broad NIR absorption, strong fluorescence, and exceptional stability, enabling precise NIR-II imaging and multimodal cancer therapy. HCY-3 J-NPs represent a promising class of stable NIR-II photosensitizers that combine imaging and therapy in a single platform. Their robustness under heat and light, strong tumor targeting, and dual PTT/PDT activity make them attractive candidates for clinical translation in cancer treatment.
- π-π-Coupled J-Aggregates Synergized With Hydrogen Bonding via Molecular Planarity Engineering for Second Near-Infrared Imaging and Phototherapy
Shuai Zhao, Shengru He, Rongrong Deng, Ping Yang, Yincheng Chang, Jun Nie, Fang Sun
Chem. Eur. J. 2025
https://doi.org/10.1002/chem.202502825