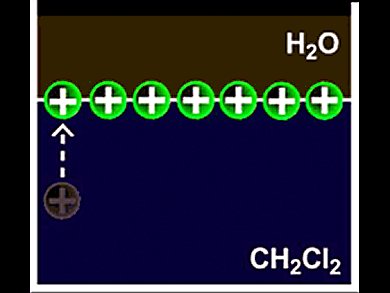
Self-assembly of nanoparticles (NPs) at liquid interfaces is considered the best bottom-up approach to fabricating multifunctional nanostructured materials with hierarchical ordering. A spontaneous arrangement of metal NPs into hexagonal, well-organized monolayer lattices is uncommon. It is only observed at curved liquid-liquid (Pickering emulsions) and air-liquid interfaces.
Volodymyr Sashuk and Marcin Fialkowski, Polish Academy of Sciences, Warsaw, and colleagues present a fully autonomous self-assembly of NPs into uniform, hexagonally packed monolayer (ML) at a planar liquid-liquid interface. This self-assembly takes place in a biphasic system consisting of an oleic dichloromethane and an aqueous phase.
Au NP’s with a mixed ligand shell, composed of ionic and hydrophobic ligands, migrate spontaneously from the bulk organic phase to form a close packed ML at the oil-water interface. The densely packed MLs were created as a result of a fine balance between the lateral repulsive electrostatic forces that prevent the NPs from aggregation and the attractive hydrophobic forces between the NPs and the oil-water interface. Further studies to gain a deeper insight into the mechanisms underlying this process are under way.
- Autonomous Self-Assembly of Ionic Nanoparticles into Hexagonally Close-Packed Lattices at a Planar Oil–Water Interface,
Volodymyr Sashuk, Robert Hołyst, Tomasz Wojciechowski, Ewa Górecka, Marcin Fiałkowski,
Chem. Eur. J. 2012.
DOI: 10.1002/chem.201103272