The plant genus Capsicum, ranging from bell peppers to chili peppers, bestows upon us a set of condiments now beloved the world over, and which allow one to “jazz up” dishes not only with respect to taste, but also visually. Hungarian, Mexican, Korean, and Indian cuisines are almost unthinkable without their peppery sting. 
But how is it that only members of the species Capsicum are able to make compounds that produce this intense effect? Here, we will try to explore the scientific background behind this sensation of “a tongue on fire” – which fades only gradually – in the hope that our readers come to enjoy spicy dishes more when they are more informed.
1. From Haiti to Europe
For aficionados of spicy dishes, January 1st should class as an important holiday (quite apart from being New Year’s Day!), because on that day in 1493, Christopher Columbus – on his first trip along the north coast of present-day Haiti – discovered a plant he assumed must be related to the common black pepper on account of the extraordinary spiciness of its fruit. “This pepper, that the local Indians use as a seasoning, grows everywhere here, and is more valuable than black pepper or melegueta pepper” [1]. The latter is often called “Grains of Paradise”.
Actually, this discovery wasn’t totally by chance, because one goal of the expedition – financed by the Spanish monarchy – was to locate a rich new source of pepper. In Europe, pepper was not the most expensive of spices, but it was quantitatively the most important, and the lucrative pepper trade was firmly in the hands of the Venetians, much to the chagrin of the Spanish court. This dominance somehow needed to be broken!
Columbus called his new plant “red pepper”, although with some uncertainty: “It causes me a great deal of pain that I am not able actually to identify it [the plant], especially because I am sure that it is valuable.” [2]
It turned out that his botanical suspicions were indeed justified. It was the French botanist Joseph Pitton de Tournefort (1656–1708) who later became the first to classify the supposed “red pepper” under the new genus Capsicum, where it became part of the nightshade family (solanaceae). Black pepper (Piper nigrum, from a long-familiar pepper-plant family, the piperaceae) is not even related to what Columbus took to be a kind of “pepper”, but his mistake proved an enduring one, as various fruits of the Capsicum family are still referred to today in everyday language as “peppers”.
The new plants that Columbus brought to Europe soon became widely distributed, even into Asia, thanks to trade along the traditional “Spice Road”. Capsicum was quickly integrated into regional cuisines of the Mediterranean countries, as well as North Africa and both the Near- and Far-East, whereas Central Europeans at first reacted skeptically to this new “pepper”, adopting the plants only as ornaments [3]. This is in a way rather astonishing, as Leonhart Fuchs made explicit reference to red pepper in his herbal book of 1543, noting that the seeds of the new Indian pepper “produced nearly all the effects, and had nearly all the virtues of real pepper”.
Capsicum eventually made its way, through the Ottoman Empire, into Hungary in the 17th century, where it quickly developed into a sort of “national spice”, and from there it slowly migrated into much of Central Europe.
2. Botanical Aspects of Capsicum
Capsicum plants were native to what is now Bolivia and Peru. Birds further disseminated the robust wild plant over large reaches of South and Central America. The indigenous peoples of these regions domesticated the plant more than 6000 years ago [4, 5], making Capsicum the very first of all plants to be cultivated by man.
 Among the over 30 wild varieties, only five have actually been domesticated: Capsicum annuum (see Fig. 1), Capsicum frutescens, Capsicum chinense, Capsicum baccatum, and Capsicum pubescens, where the first three are the ones that have gained economic significance. The names they have been assigned are puzzling: Capsicum annuum is most certainly not an “annual”, the fruit of C. baccatum is not limited to “berries”, Capsicum chinense has its origins in the Caribbean, not China, and the leaves of C. pubescens are not always “hairy”! The boundaries between the various species are also subject to hefty dispute among botanists. For example, many authors combine C. chinense and C. frutescens together in a single class. Unquestionably, at the head of the pack is Capsicum annuum, and for one simple reason: quite accidentally, this was the variety Columbus encountered in Haiti, and then brought home with him to Europe.
Among the over 30 wild varieties, only five have actually been domesticated: Capsicum annuum (see Fig. 1), Capsicum frutescens, Capsicum chinense, Capsicum baccatum, and Capsicum pubescens, where the first three are the ones that have gained economic significance. The names they have been assigned are puzzling: Capsicum annuum is most certainly not an “annual”, the fruit of C. baccatum is not limited to “berries”, Capsicum chinense has its origins in the Caribbean, not China, and the leaves of C. pubescens are not always “hairy”! The boundaries between the various species are also subject to hefty dispute among botanists. For example, many authors combine C. chinense and C. frutescens together in a single class. Unquestionably, at the head of the pack is Capsicum annuum, and for one simple reason: quite accidentally, this was the variety Columbus encountered in Haiti, and then brought home with him to Europe.

Figure 1. Capsicum annuum species.
The layman would find it almost impossible to assign a particular plant to its proper species, and even botanists disagree about the number of species, their distinguishing characteristics, and the boundaries that separate the various peppers [6].
As a consequence of centuries-long cultivation in diverse climatic zones, and the frequent identification of new cultivars, the five species mask an enormous number of subspecies, types, and varieties, bred intentionally with respect to criteria such as robustness, harvest yield, intensity of color, pungency (“hotness”), and aroma.
The result is also a linguistic tangle of Babylonian proportions. Consider: what the Germans call “Paprikaschoten” (pepper pods) – although from a strictly botanical perspective these are not pods at all, but instead hollow berries, as this indehiscent fruit (a type that at maturity does not open to discharge seeds) is formed from a single ovary, and the pericarp (fruit wall) of the ripe fruit is juicy and meaty – or “Gemüsepaprikas” (vegetable peppers), are known in many English-speaking countries simply as peppers. But in many places, including the United States, also as bell peppers, due to their shape. To add to the mix, in Switzerland the same things are called Peperoni, but in Austria Pfefferoni are “hot” peppers, which the Germans call Peperoni. In Italian and English, “pepperoni” refers to spicy sausages. In parts of Latin America, spicy (hot) peppers are aji, in southern Africa they are piri-piri, and in many countries chilis. The latter go under the name chili peppers in the USA, but simply chiles in Mexico. To further complicate things: especially in certain southwestern states in the USA, “chili” may indeed be associated with a spicy version of the fruit, but it more often designates a sort of “bean stew”. Chilli (with two “l’s”) in England and Australia means a “hot” variety of Capsicum, which ever more frequently in Germany is also called either Chilli or simply Chili, but sometimes Peperoni.
From a botanical standpoint the whole thing is far simpler: all are fruits belonging to the species Capsicum – “Paprika” in German, and “pepper” in English. Period!
3. Culinary Matters Relative to Peppers
3.1 Their Splendid Color
When we enjoy peppers, whether raw or in some elaborate culinary creation, our pleasure is always the consequence of a harmonic interplay involving all our senses. Our eyes capture the beautiful form and color, our ears the crackle and consistency as we chew, our tongues the flavor components (sweet, sour, salty, bitter, and umami [7, 8]), and our noses the bouquet or scent.
Now let us consider these remarkable sensory aspects of peppers from a chemical point of view. You’ll be amazed!
The green, yellow, orange–red, or deep red bell peppers are especially attractive visually, and for that reason alone they are prized in virtually every cuisine. As it ripens, a pepper puts on a kaleidoscopic display, passing sequentially from bold green by way of yellow and orange to a deep red (see Fig. 2). This, of course, reflects a coordinated synthetic chemical process, which we will look at a bit more closely in what follows.

Figure 2. The play of colors at various stages during the ripening of C. annuum cv. Charleston Hot.
The Immature, “Green” State
As with other fruits, the principal pigments visible to us early on are the chlorophylls a and b ((1a) and (1b); Fig. 3), probably most familiar as the universal leaf pigments. Chlorophylls are complex macrocyclic compounds with an extensive system of conjugated double bonds, responsible in turn for conferring upon the molecule its color. One side chain, bonded by way of an ester linkage, represents a C20 alcohol (phytol), through which the pigment is anchored in the thylakoid membranes of the chloroplasts. Chlorophyll is at the very heart of photosynthesis, though it is far from the only pigment involved; several yellow and yellow-orange carotenoid pigments also act as gatherers of light.
.jpg)
Figure 3. Sources of color in peppers.
The term “carotenoid” encompasses not only the carotenes (C20 hydrocarbons), but also their oxygenated derivatives, the xanthophylls. However, the yellow color displayed by these latter pigments generally remains hidden to the eye as in most circumstances the chlorophylls completely mask all other colors present.
The Ripening Process
As the fruit ripens, chlorophylls – together with the secondary photosynthesis pigments – are slowly decomposed, and new yellow and ultimately red pigments emerge [9]. Figure 4 presents an overview of the pigment content of three C. annuum varieties before and after maturation. The changes in pigmentation correspond to the play of colors depicted in Figure 2. A conspicuous feature is nearly complete degradation of the yellow–orange lutein (4), and the synthesis of the deep-red pigments capsanthin (8), capsanthin-5,6-epoxide (9), and capsorubin (10).
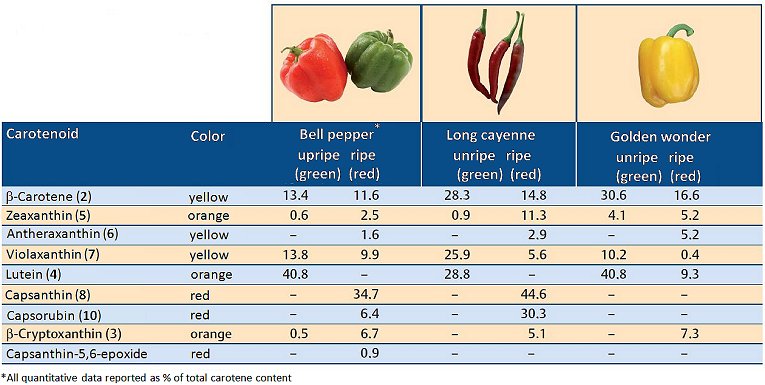
Figure 4. Carotenoids in unripe and ripe peppers (C. annuum) [10].
The enjoyment of the brilliant colors can be increased by learning that peppers are one of the very few plants capable of biosynthesizing these red pigments, which are, therefore, sometimes called the “paprika ketones” (note the presence of a keto function in each). Animals are incapable of synthesizing any carotenoids. Though some make use of those they consume from vegetation in their diets – using them either directly or after chemical modification. Thus, hens employ carotenoids in the production of yellow egg yolks, flamingos need them for their rose-colored plumage, and the characteristic hue of salmon flesh also depends on carotenoids salmon find in their diet. Finally, the Egyptian vulture (Neophron percnopterus) owes the unique yellow color of its face to a particularly exotic diet; it subsists largely on the dung of herbivorous animals [11–14]!
“Paprika Ketones“
Biosynthesis of the “paprika ketones” capsanthin and capsorubin is remarkable as it involves an unusual pinacol rearrangement (see Fig. 5) [15].
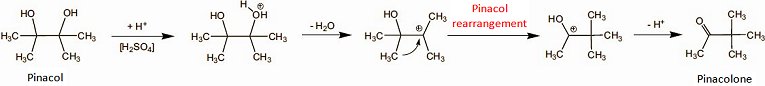
Figure 5. The pinacol–pinacolone rearrangement.
The chemically versed gastronome should savor the extraordinary occurrence of this reaction in the mouth, because in the laboratory a pinacol rearrangement typically requires the presence of concentrated sulfuric acid, but peppers achieve it quite effortlessly at pH 7 and room temperature (albeit with help from a hitherto unknown enzyme system; Fig. 6). Hats off!
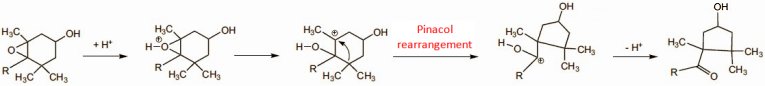
Figure 6. Biosynthesis of the pepper ketones.
Degradation of the green chlorophylls supports the biosynthesis of the carotenoids, which entails the incorporation of eight isoprene units. The initially formed hydrocarbons, e.g., β-carotene itself, are subsequently oxidized to alcohols or epoxides. Biochemical studies with 14C- and 3H-labeled compounds have made it possible to show that the paprika ketones arise exclusively and directly, through pinacol rearrangements, from the epoxides antheraxanthin (6) and violaxanthin (7); (see Fig. 7) [26–28].
.jpg)
Figure 7. The biosynthesis of pepper ketones; the pepper ketones are formed exclusively and directly through pinacol rearrangements, starting with the epoxides antheraxanthin and violaxanthan.
3.2 The Seductive Aroma
With respect to culinary pleasure, only rarely is a rigorous distinction made between taste and smell, as awareness of both derives from the nose/mouth realm, and both contribute to the overall sensation we describe as “flavor” or “taste”. Eating with a congested nose, or with the nose pinched shut, provides convincing proof that the “taste” of a particular food is perceived, to a great extent, by the nose in the form of an aroma, and rather less as a flavor, detected with the tongue.
For most of the (red) pepper grown throughout the world it is actually an appealing aroma that matters most, not how “hot” the product is. For this reason, a great many studies have been conducted of the various characteristic scents. Surprisingly, the aroma so familiar and typical for a green bell pepper is due almost entirely to a single substance: 2-methoxy-3-isobutylpyrazine (11); (see Fig. 8). The human nose is able to detect this “pepper-scented” compound at the almost unbelievably dilute aqueous concentration of 0.002 parts-per-trillion (i.e., 2:1,000,000,000,000). This means that the so-called bell pepper pyrazine is, for humans, among the very most odor-intense substances known.
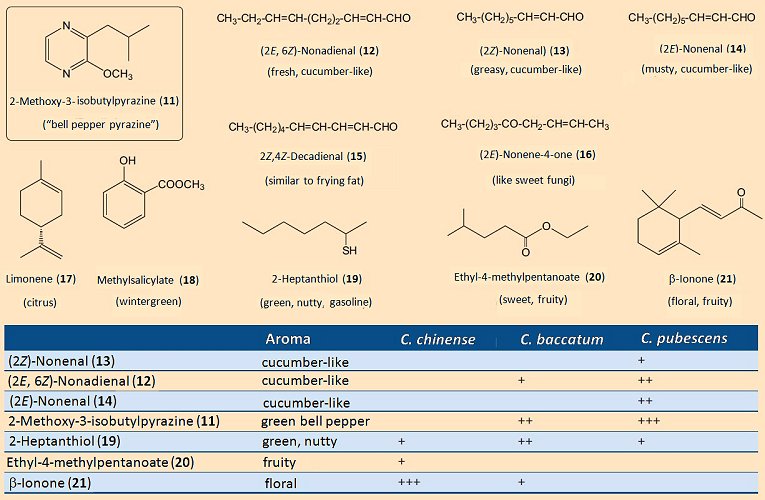
Figure 8. Aromatic (fragrant) components in peppers.
Apart from this pyrazine, there are a few other compounds that contribute to the overall characteristic aroma of a pepper (see Fig. 8). It is of culinary importance, too, that the various pepper species exhibit significant differences in their aroma profiles. For example, among the volatile components of C. chinense there is no bell pepper pyrazine (11) whatsoever, so that here the typical aroma of green pepper is completely absent. In its place is β-ionone (21), as well as various esters, all of which lead to a corresponding fruity/floral bouquet.
C. pubescens presents a supplementary light, nutty scent, resulting from 2-heptanthiol (19). A mixture of short-chain esters, e.g., (20), is what gives the Tabasco variety of C. frutescens its powerful and characteristic fruity aroma [16].
As a result of the wide range of varieties, as well as the diverse growing conditions represented by the many cultivation regions around the world, the information about the aroma given above only provides a guide. Indeed, one of the charms of the pepper family is the fact that the culinary pleasure to be derived is ascertainable neither from size, nor color, nor place of origin. In other words, more than ample room is available for surprise.
3.3 The Spicy (“hot”) Components
What most clearly distinguishes pepper plants and their fruits from all other species is the extent of their spiciness. It is true that ginger, mustard, and black pepper also count among what we regard as ”strong” seasonings, but when it comes to absolute “sting” or “hotness”, nothing approaches the red pepper. It is no wonder, then, that the agents responsible for “hotness” are produced uniquely by the single species Capsicum. From the standpoint of synthetic creativity, none of their close relatives within the nightshade family can surpass the peppers. We learned long ago to keep at arm’s length henbane, belladonna, angel’s trumpet, jimsonweed, and even tobacco, but the related Capsicum simply regales us with the effects of its unique alkaloids.
Synthesis
It is understandable that chemists have for ages been interested in the spicy components of peppers. Their first isolation was accomplished in 1876 by Thresh [17], who was also first to put forth the name “capsaicin”. In all probability, however, the material he had was actually a mixture. Structure 22 (see Fig. 9) was first proposed for the principal component by Nelson in 1919 [18], a structure that has since been confirmed in various ways through many total syntheses [19–21].
.jpg)
Figure 9. The spicy (“hot”) substances in peppers. Scoville heat units = SHU.
Given the compound’s relatively simple structure, it has also become possible to prepare a host of derivatives; pharmacological studies have been conducted as well in a quest for definitive structure–activity relationships. Thus, the vanillyl-like residue – including its amide function group – is now known to be essential, whereas anywhere between 8 and 11 carbon atoms can be present in the carboxyl chain. Shorter or longer chains do reduce the pungency somewhat, whereas terminal methyl branching clearly enhances it and the presence or absence of the double bond makes only a minimal difference.
The various pepper species and their countless varieties differ not only in overall content of “hot” substances, but also in the distribution of the various contributors [22]. So far, over 20 different capsaicinoids are known, in all of which an unchanging aromatic vanillyl-like residue (red; Figs. 9 and 10) is joined through an amide linkage to an aliphatic chain (black).
From among the many relevant compounds, capsaicin itself (22) and dihydrocapsaicin (24) always constitute over 80 % of the capsaicinoids present in all the hot peppers studied. These two compounds also produce the greatest sensory impact [23], and, thus, their content also determines the overall pungency of a pepper. Nevertheless, minor components also make a contribution to the overall pleasure conferred, owing to their diverse sensory characteristics.
The ratio of capsaicin to dihydrocapsaicin is species specific: 1:0.8 in C. annuum, 2:1 to 1:1.5 in C. baccatum, 2.5:1 in C. chinense/frutescens, and 1:1.5 in C. pubescens.
The pungent oral sensation induced by capsaicin (22) and dihydrocapsaicin (24) increases rapidly in the middle and rear portions of the tongue and gums, and persists for a long time, whereas for homodihydrocapsaicin (25) the effect develops more slowly, and only in the rear of the mouth. Nordihydrocapsaicin (23) is perceived as milder in the frontal region of the tongue, and its effect diminishes more rapidly.
How does capsicum – the only species that manages to do so – synthesize these wonderful pungent substances?
Studies with isotopically labeled metabolites have shown that the amino acid phenylalanine is the starting point for the aromatic portion of all these molecules, with the various carboxylic acids segments coming from the amino acids valine, leucine, or isoleucine, depending upon chain length and branching pattern (see Fig. 10). The enzyme capsaicin synthase joins the two fragments by way of an amide linkage in the final synthetic step. Capsaicin synthase was reportedly isolated from the placenta of Capsicum, and found to be a protein with a molecular weight of 38 kDa, the synthetic activity of which correlated well with the concentration of capsaicin in the tissue [24].
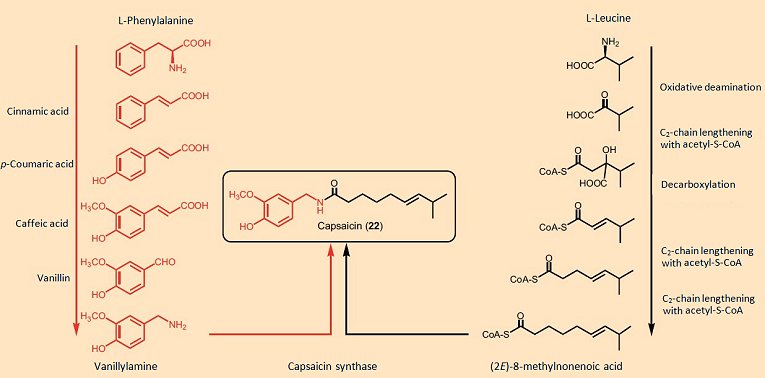
Figure 10. Biosynthesis of capsaicin.
Unfortunately, subsequent to publication of these results, it turned out that the protein investigated was perhaps not a “capsaicin synthase” after all, but could have instead been a protein kinase, and two years later the paper was withdrawn [25]. The true structure of capsaicin synthase, therefore, remains unknown.
One can use the tongue to test where capsaicin synthesis takes place in spicy varieties of the peppery fruit. Contrary to commonly held belief, the seeds are not a source of capsaicin. Instead, capsaicin-producing glands are to be found in the upper layer of the placenta (see Fig. 11). At very high capsaicin concentrations, however, the compound can diffuse into neighboring tissue [26–28]. A similar migration also frequently occurs in the course of processing and drying.
.jpg)
Figure 11. Where does the actual “hotness” (pungent spiciness) originate in peppers?
Bell pepper pyrazine (11), which produces the characteristic flavor and scent in green bell peppers, is found primarily in the fleshy parts of the fruit. Some varieties of C. chinense, including Scotch Bonnet and Habanero, contain essentially nobell pepper pyrazine, so that with these especially “hot” varieties the typical “vegetable pepper aroma” is completely absent, replaced instead by a strong fruity character [29, 30].
The components constituting the overall bouquet are synthesized in various parts of the fruit, where bell pepper pyrazine (11), source of the characteristic green bell pepper scent, originates in the flesh, whereas fruity and floral esters come from the placenta.
In other words, “hot” spiciness and aroma are most certainly not mutually exclusive.
References
[1] J. Andrews, The Pepper Trail: History and Recipes from Around the World, The University of North Texas Press, Denton, TX, USA, 1999. ISBN: 978-1574410709
Melegueta pepper (Aframomum melegueta) is a West African ginger plant whose seeds (“grains of paradise“) are prized as a pungent spice.
[2] A. Dalby, Dangerous Tastes, British Museum Press, London, 2000. ISBN: 9780714127200
[3] E. Vaupel, Gewürze, Deutsches Museum, Munich, Germany, 2002. Link
[4] Linda Perry, Ruth Dickau, Sonia Zarrillo, Irene Holst, Deborah M. Pearsall, Dolores R. Piperno, Mary Jane Berman, Richard G. Cooke, Kurt Rademaker, Anthony J. Ranere, J. Scott Raymond, Daniel H. Sandweiss, Franz Scaramelli, Kay Tarble, James A. Zeidler, Science 2007, 315, 986–988. DOI: 10.1126/science.1136914
[5] L. Perry, K. V. Flannery, Proc. Nat. Acad. Sci. 2007, 104, 11905–11909. DOI: 10.1073/pnas.0704936104
[6] C. B. Heiser, Jr., Of Plants and People, University of Oklahoma Press, Norman, USA, 1992. ISBN: 9780806124100
[7] Jayaram Chandrashekar, Mark A. Hoon, Nicholas J. P. Ryba, Charles S. Zuker, Nature 2006, 444, 288–294. DOI: 10.1038/nature05401
[8] S. Yamaguchi, K. Ninomiya, J. Nutr. 2000, 130, 921. Link
[9] B. Camara, R. Monegar, Phytochemistry 1978, 17, 91–93. DOI: 10.1016/S0031-9422(00)89686-7
[10] V. S. Govindarajan, CRC Crit. Rev. Food Sci. Nutr. 1986, 24, 245.
[11] K. Roth, Chem. Unserer Zeit 2009, 43, 100–114. DOI: 10.1002/ciuz.200900485
[12] M. Ratermann, Chemkon 2001, 8, 149–153. DOI: 10.1002/ckon.20010080307
[13] K. Meyer, Chem. Unserer Zeit 2002, 36, 178. DOI: 10.1002/1521-3781(200206)36:3<178::AID-CIUZ178>3.0.CO;2-#
[14] J. J. Negro, J. M. Grande, J. L. Tella, J. Garrido, D. Hornero, J. A. Donázar, J. A. Sanchez-Zapata, J. R. BenÍtez, M. Barcell, Nature 2002, 416, 807–808. DOI: 10.1038/416807a
[15] Stefan F. Kirsch, Nachr. Chemie 2008, 56, 1228–1231. DOI: 10.1002/nadc.200860989
[16] L. Wendell. Haymon, Leonard W. Aurand, J. Agric. Food Chem. 1971, 19, 1131–1134. DOI: 10.1021/jf60178a027
[17] M. Thresh, Pharm. J. Trans. 1876, 7.
[18] E. K. Nelson, J. Am. Chem. Soc. 1919, 41, 1115–1121. DOI: 10.1021/ja02228a011
[19] L. Crombie, S. H. Dandegaonker, K. B. Simpson, J. Chem. Soc. 1955, 1025-1027. DOI: 10.1039/JR9550001025
[20] Peter M. Gannett, Donald L. Nagel, Pam J. Reilly, Terence Lawson, Jody Sharpe, Bela Toth, J. Org. Chem. 1988, 53, 1064–1071. DOI: 10.1021/jo00240a024
[21] Harumi Kaga , Masakatsu Miura , Kazuhiko Orito, J. Org. Chem. 1989, 54, 3477–3478. DOI: 10.1021/jo00275a040
[22] J. Jurenitsch, U. Kastner, Pharm. Unserer Zeit 1994, 23, 93. DOI: 10.1002/pauz.19940230208
[23] P. H. Todd, Jr., M. G. Bensinger, T. Biftu, J. Food Sci. 1977, 42, 660. DOI: 10.1111/j.1365-2621.1977.tb12573.x
[24] B. C. Narasimha Prasad, Vinod Kumar, H. B. Gururaj, R. Parimalan, P. Giridhar, G. A. Ravishankar, Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 13315–13320. DOI: 10.1073/pnas.0605805103
[25] B. C. N. Prasad et al., Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 20558.
[26] B. Camara, FEBS Letters 1980, 118, 315–318. DOI: 10.1016/0014-5793(80)80247-X
[27] B. Camara, Biochem. Biophys. Res. Commun. 1980, 93, 113–117. DOI: 10.1016/S0006-291X(80)80253-1
[28] B. Camara, R. Moneger, Biochem. Biophys. Res. Commun. 1981, 99, 1117–1122. DOI: 10.1016/0006-291X(81)90734-8
[29] P. W. Bosland, E. J. Votava, Peppers: Vegetable and Spice Capsicums, CABI Publishing, Wallingford, UK, 2000. ISBN: 0851993354
[30] H. Kollmannsberger, Dissertation TU München, Germany, 2007.
The article has been published in German in:Chem. Unserer Zeit 2010, 44(2), 138–151. DOI: 10.1002/ciuz.201000525
and was translated by W. E. Russey.
The Biochemistry of Peppers – Part 2
If a bowl of chili con carne “tastes” good because of its pleasant sharpness, this is, strictly speaking, wrong. We can only taste sweet, bitter, sour, salty, and umami. Sharp is not among them!
► See all articles by Klaus Roth published by ChemistryViews magazine




