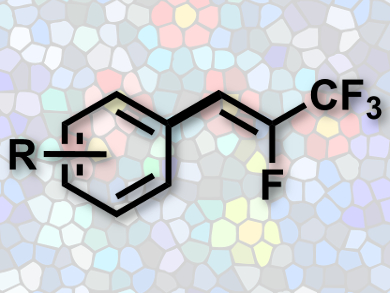
Fluorinated compounds are of great interest to medicinal chemistry, materials sciences and agrochemistry. Conjugated aromatic systems with trifluoromethyl groups such as β-(trifluoromethyl)styrene derivatives have found wide use in organic light-emitting diodes and in other applications of materials chemistry. The synthesis of these compounds is difficult, however.
Zhao-Tie Liu, Shaanix Normal University, Xi’an, China, Jian Lu, Xi’an Modern Chemistry Research Institute, China, and colleagues have developed a facile synthesis of (Z)-β-(trifluoromethyl)styrene derivatives (pictured). Commonly available 2,3,3,3-tetrafluoroprop-1-ene was used as a fluorine source in a palladium-catalyzed oxidative Heck reaction.
A large number of important arylboronic acids can be coupled with fluorinated olefins. This makes the method practical for the streamlined synthesis of functionalized styrenes.
- Oxidative Heck Reaction of Fluorinated Olefins with Arylboronic Acids by Palladium Catalysis,
Yang Li, Dong-Huai Tu, Yu-Jie Gu, Bo Wang, Yao-Yu Wang, Zhao-Tie Liu, Zhong-Wen Liu, Jian Lu,
Eur. J. Org. Chem. 2015.
DOI: 10.1002/ejoc.201500597