Summer 1878: two chemists discover, quite unexpectedly, an extremely sweet compound, shown later to be utterly non-toxic, as well.
But instead of this being the start of a chemical success story centered around an inexpensive sugar substitute, it signaled instead the beginning of a turbulent back and forth in a story line determined by a life-long priority struggle, groups of economic stakeholders, tax law, the market, wild bands of smugglers, and the spirit of the times. Curiosities, tragedy, jealousy, passion, and thrilling chemistry all run their course. Take your seats! The curtain rises!
1. Prologue: Our Insatiable Quest for All Things Sweet
Mankind loves sweet foods! No wonder, since over tens of thousands of years this taste sensation helped our early forefathers – hunter/gatherers – in finding nourishment that was rich in calories. For stone-age man, who was kept fully engaged by the search for sustenance, a taste for sweet things represented a definite evolutionary advantage. Sweet honey, sweet fruits and roots – all these signaled high nutritional value.
1.1. Cane Sugar
Naturally occurring sugar was first isolated in pure form by the Persians around 600 A.D.: through evaporating aqueous extracts from East Asian sugar cane (Saccharum officinarum). In fact, cane sugar was the very first organic natural product mankind managed to isolate in pure crystalline form.
Arabic traders introduced this exotic specialty into Europe in the early Middle Ages, where it was subsequently dispensed exclusively in pharmacies, either as a high-priced remedy or as a costly condiment. Sweet foods and drinks remained a privilege restricted to the affluent for centuries. While it is true that cane sugar became more readily available in the 16th century as a result of flourishing overseas trade – brought about in turn by recent colonial conquests – it still remained out of reach for most people [1].
1.2. Beet Sugar
As late as the 19th century, the sugar market owed its prosperity to Great Britain and the other great colonial powers. It is no wonder that resourceful heads elsewhere long sought a new plant source of sugar, one capable of thriving in central Europe. In 1747, Andreas Sigismund Marggraf, a member of the Royal Academy of Berlin, Germany, finally recognized such a species in the beet Beta vulgaris, from which he was able to isolate “a true, perfect sugar, entirely like that sugar derived from common sugar cane”.
However, it was left to his disciple and successor at the Royal Academy, Franz Carl Achard, to raise the sugar content in the beet from 1.6 % to 5 % [2,3]. When Achard presented the Prussian King Friedrich Wilhelm III with a first sample of his “beet sugar”, he was rewarded with 50,000 Taler, with which he was able to purchase an estate in Kunern in Silesia (now Poland). There he cultivated sugar beets, erecting the first beet-sugar factory in the world in 1801. The cane sugar monopoly was thus broken.
One specific disaccharide, sucrose or O-α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside, is referred to in everyday speech as “sugar”. If desired, one can also indicate a source, as “cane” or “beet sugar”, though the two are chemically the same.
1.3. The Sugar Industry
Sugar factories, with their huge extraction towers, lime kilns, evaporative stations, centrifuges, and sugar silos, represented the first industrial agricultural facilities in the world. Under protection of the Continental Blockade, first declared in Berlin by Napoleon on 21 November 1806, the young European beet sugar industry quickly flourished, since this decree, directed against the economic blockade established by England, forbade all importation of cane sugar from abroad [4].
When, after Napoleon’s downfall in 1814, the continental blockade was rescinded, cheap cane sugar poured into central Europe and things quickly went downhill for the beet-sugar industry, which was not yet in a position to compete on its own. Only when protected by high import duties and after comprehensive technical improvements did the beet-sugar industry begin to recover, and by the middle of the 19th century it found itself in the vanguard of the industrial revolution. Indeed, it was its engine: By 1840, 22 % of the sugar consumed in the German Empire was derived from beets, by 1850, 50 %, and indeed by 1880,100 %. Sugar became less expensive, and affordable by broader strata of the population, and as a consequence, sugar consumption rose.
Starting in 1841, the public coffers compensated for a dramatic decrease in income from sugar import duties through a sugar tax on domestic beet sugar. Increasing sugar consumption caused inland revenue to climb so much that the sugar tax alone amounted to 7 – 10 % of the overall individual tax load, the largest of the individual tax items in the entire budget for the German Empire, and thus a mainstay in the state budget. Political influence on the part of the sugar industry was correspondingly powerful. Appropriately, the “Association for the Beet Sugar Industry in the Tariff Union”, founded in 1850, became the very first German trade association.
Pure granulated sugar was and is not essential for our sustenance, because thanks to starch-containing grain and vegetables there are ample inexpensive domestic sources of starch throughout the world. In other words, sugar remains a semiluxury food, hence a favorite of treasury secretaries or finance ministers everywhere. After all, luxury items are easy to tax (e.g., tobacco, alcoholic beverages, coffee, etc.). And once levied, such taxes are hard to abolish, so the German sugar tax has enjoyed a long life. The sugar tax, originally introduced in Prussia in 1841, was later adopted by the German Empire as a whole, and then by the Federal Republic, and only done away with in the context of “tax harmonization” for the European Union (EU) in 1994.
Irrespective of whether cane sugar or beet sugar, sucrose (once known as “saccharose”) had no serious competitors as a sweetening agent. Honey was always more expensive due to the elaborate isolation process. This all changed in June of 1878, however, when a potential competitor did slip onto the stage, initially quite unnoticed. A profound drama full of emotion and gaping human abysses had begun.
2. Saccharin First Glimpses the Light of Day – from a Laboratory [5]
In 1877, the firm of William H. Perot & Co., located in Baltimore, MD, USA, hired one Constantin Fahlberg (see Fig. 1), a German chemist residing in New York, to act as an expert witness in a suit brought against the United States over confiscation of 712 sacks of demerara sugar. Demerara sugar is a coarse, crystalline, brown form of sugar with a 2 – 3 % molasses content, once produced primarily in Demerara (now a part of Guyana, long known as British Guiana).
This material was alleged to have been artificially darkened so it would fall in a lower category, and thus be subject to a reduced import duty. The government’s suspicion was shown to be baseless in the course of legal proceedings, however. Fahlberg was to carry out his advisory sugar analyses in the laboratory of Ira Remsen (see Fig. 2) at Johns Hopkins University in Baltimore.
|
|
|
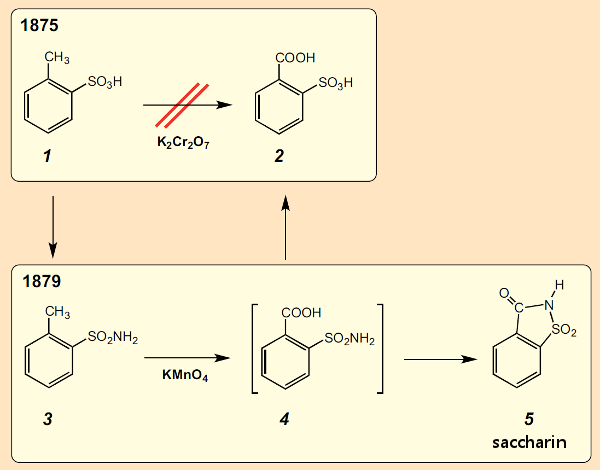
The start of the trial was delayed, and when Fahlberg had finished his analyses he asked Remsen whether he might work as part of the latter’s research group until proceedings actually began. Remsen agreed, and suggested that Fahlberg carry out a closer study of the oxidation of o-toluenesulfonic acid (1) (see Fig. 3). Remsen’s first attempts a few years earlier to perform the oxidation using potassium dichromate in hopes of getting directly to o-sulfobenzoic acid (2) had been unsuccessful [6].
Working with the doctoral candidate M. W. Iles, Remsen had been able to show, however, that ortho methyl groups in aromatic sulfonamides could be oxidized cleanly with potassium permanganate, so he revised his synthetic plan for 1 [7]. This detour via the sulfonamide led to success: Fahlberg was indeed able to isolate the target 2, albeit as a byproduct (see Fig. 3). The main product was compound 5, designated at the time as “anhydroorthosulfamine benzoic acid”.
|
|
|
Figure 3. The chance discovery of saccharin. |
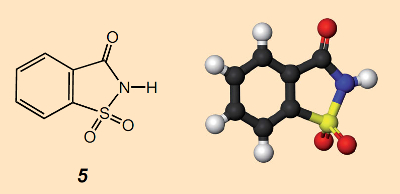
Later, this “anhydroorthosulfamine benzoic acid” 5 was renamed by Fahlberg and Remsen to “benzoic acid sulfinide”. The correct IUPAC name today is 1,1-dioxo-1,2-benzothiazol-3-one. The common trade name introduced several years later for the substance, “saccharin”, is generally employed, as it will be in this article as well.
Though no sensation at the time, two brief and parenthetical sentences in the publication “Über die Oxidation des Orthotoluolsulfamids” [8], submitted in February of 1879, came to signal the birth of the first artificial sweetener (see Fig. 4, Tab. 1): “It tastes pleasantly sweet; indeed, sweeter than cane sugar. Even in very dilute solution its presence is easily recognized by taste.”
|
|
|
Figure 4. Profile for saccharin, 1,1-dioxo-1,2-benzothiazol-3-one. |
The way this discovery actually occurred was described later by Fahlberg [9]:
|
“After working diligently all day in Baltimore in the Johns Hopkins University laboratory, and before going home that evening, I washed my hands thoroughly, thinking in the process I had done my duty completely. I was very startled to find at dinner, however, as I put some bread in my mouth, that my hands tasted sweet. I was at first suspicious that the housekeeper had unexpectedly sweetened the bread itself, so I asked her about it. A bit of a debate ensued, which she ultimately won. It turned out that it wasn’t the bread that tasted sweet, but rather my hands, which I knew I had washed; I was amazed that, after further direct contact with my tongue, I had to admit that not only both hands, but also both my arms tasted sweet! No other circumstance could have been involved here than that, despite my washing, I had somehow brought the taste home from my work at the laboratory. I went back to the laboratory and tasted all my beakers, glasses, and bowls that I’d left standing around on the lab bench, until finally I encountered the taste of some contents that seemed to me to be of astonishing sweetness. In any event, discovery of the material with eminent sweetening power had thereby occurred.” |
The tasting of flask and beaker contents of uncertain nature would today be considered unusual at the very least, but it does have a tradition in chemistry, which is a close relative of pharmacy. The sense of taste is a very sensitive one, and able to make very subtle distinctions.
The great Emil Fischer, toward the end of the 19th century, tasted all the sugars synthesized in his laboratory. His poor doctoral candidates were often forced to watch helplessly as crumbs of material, the preparation and purification of which represented weeks of work, disappeared into Fischer’s mouth, never to be seen again. Fearless pharmacists distinguished between the two extremely bitter natural substances quinine and strychnine by their aftertaste until the middle of the 20th century: the bitterness of quinine disappeared rather quickly, while that of strychnine would typically survive an entire luncheon.
Ira Remsen would almost certainly have approved of the account above, but would perhaps have added the fact that 75 of his total of 158 publications dealt with the chemistry of aromatic sulfonic acids. When Fahlberg joined his research group, the latter had no previous experience in this field, so it was necessary for Remsen to “tell him exactly what he was to do, and he (Remsen) would also discuss with him (Fahlberg) from beginning to end the work to be carried out that day, so that every step involved in the result occurred under his (Remsen’s) personal direction” [5].
Remsen was very generous with respect to authorship. Thus, early in 1878, a doctoral candidate of his, Iles, was allowed to publish a paper in Berichte der deutschen chemischen Gesellschaft along with Fahlberg in which an improved method for determining sulfur content was tested with various sulfonic acids from Remsen’s research group. Remsen himself passed up the right to co-authorship. Even today that would be an exceptionally genial and far from matter-of-course gesture on the part of a university professor.
Remsen would also point out that, in 1880 in the comprehensive publication “On the Oxidation of Orthotoluenesulphamide” [10], in which all experimental details were described, the sequence of the authors (“I. Remsen and C. Fahlberg”) was intended to make clear to whom the basic ideas in the paper should be attributed.
All in all, apart from certain nuances, assertions of the two researchers matched as they went their separate ways in June of 1880. Fahlberg left Johns Hopkins University and took a new position with the Harrison Bros. & Co. sugar refinery in Philadelphia, PA, USA.
|
Table 1. Maximal saccharin content of different foodstuffs. |
||||||||||||||||||||||||
|
3. Quarrel About Paternity
Fahlberg didn’t let go of “anhydroorthosulfamine benzoic acid” (5) even after leaving Remsen’s laboratory, and the idea of a commercial application probably occurred to him quite soon. Transformation of a substance developed in the laboratory without regard to expense or effort into a marketable product required, apart from perseverance and costly research effort, above all good business sense. In the summer of 1882, Fahlberg visited his uncle in Germany, the successful Leipzig merchant Adolph List (1823–1885), and together they developed first ideas about a possible industrial use.
Immediately upon his return, and while still with Harrison Bros. & Comp., Fahlberg began tolerance studies involving rabbits and dogs. It turned out that saccharin was eliminated almost completely (and unchanged) in the urine. In an experiment on himself, Fahlberg verified that the same also applied to humans: “I remember once in the course of a day consuming 10 g of saccharin, and on the following day, apart from a small fraction, eliminating this same amount again through the urine.”
Parallel to this, Fahlberg optimized an industrial synthesis from the standpoint of yield and byproducts. On his next visit to Leipzig, in the summer of 1884, a final decision was reached to start production. Fahlberg and his uncle Adolph List applied for patents in Germany, France, Belgium, and the USA (see Fig. 5). The patents did not pertain to saccharin itself, since a laboratory synthesis of this substance had already been made public in 1879 in Berichte der deutschen chemischen Gesellschaft. The patents instead focused on an improved industrial scale synthetic procedure, which, however, corresponded in its chemical fundamentals to those in the publication with Remsen.
|
|
|
Figure 5. Fahlberg’s German and US patent applications. |
After returning in the fall of 1884, Fahlberg resigned from Harrison Bros. & Comp., moved to New York City, and established a small pilot plant at 117th Street and the East River. With only one employee, saccharin was produced here on a scale of 5 kg per day, which was used for more animal studies at scientific institutions. After that, potential customers were provided with samples, and starting in 1885, Fahlberg was introducing his new artificial sweetener at international trade fairs, where it was frequently honored. In 1886, Fahlberg had the name “saccharin” protected through the commercial registry. The name itself was derived from the Latin “saccharum” for sugar. Fahlberg saw himself as the inventor, and in retrospect declared quite firmly: “With the name ‘saccharin’ I am providing a formal designation for a substance discovered by me in June 1878”.
Fahlberg had never informed Remsen about the patent, nor about the anticipated commercialization of saccharin, let alone asked his permission. It was not until the early summer of 1886 that Remsen found out about Fahlberg’s activities, and he was justifiably livid. Remsen protested immediately against Fahlberg’s deception in the American Chemical Journal [11]:
|
“This substance has recently come into some prominence under the name “saccharin,” which is given to it on account of its sweet taste. In the notices of saccharin, even in scientific journals, the statement is constantly made that the substance was discovered by Fahlberg. The statement needs modification. As a matter of fact, the substance came to light in the course of an investigation which Fahlberg undertook at my suggestion, and carried on under my direction, and it was first described in a paper by myself and Fahlberg which appeared in the Berichte der deutschen chemischen Gesellschaft.” |
A few months later he would make his point even more clearly in a communication to the Berichte der deutschen chemischen Gesellschaft [12]:
|
“Since it appears that there is a misunderstanding in certain circles regarding the discovery of benzoic acid sulfinide (so-called saccharin), allow me to provide the following clarification: This substance was discovered in the course of a study Fahlberg undertook a few years ago at my instigation. The study was part of a larger investigation into the “Oxidation of Aromatic Substitution Products” that I then had underway. The sulfinide was first described in a paper I sent to the Editors of this journal, and it later appeared in a larger paper in the American Chemical Journal, edited by me. Since then I have arranged for numerous experiments in order to improve the yield of the sulfinide, in the hope that the substance could be obtained in larger quantity for investigation, and although the method of preparation is so far not satisfying, it has been possible for me to carry out the experiments mentioned above. The expression “Fahlberg’s saccharin” is completely unwarranted, and hopefully will not appear again in the future. The only possible legitimacy is perhaps the fact that Fahlberg has patented the substance, without first discussing the matter with me. This fact requires no commentary.” |
Remsen resented especially Fahlberg’s denial of Remsen’s scientific contribution to the discovery of saccharin, and never forgave him for that. Shortly before his retirement he said to a student “I did not want his [Fahlberg’s] money, but I did feel that I ought to have received a little credit for the discovery” [13].
It is certainly true that, along with a large portion of luck, Remsen and Fahlberg both contributed to the discovery of saccharin, and neither would have been successful without the other. The industrial implementation is indisputably to Fahlberg’s credit, however, his concealment of his plans, and the secretiveness with respect to Remsen regarding patenting, were morally dubious and incomprehensible, since Fahlberg knew for sure that Remsen was not at all interested in the commercialization of research results, and certainly would have agreed to Fahlberg developing a commercial application. All that mattered to Remsen was scientific recognition, which Fahlberg had denied him, and he presented his viewpoint in a nutshell: “Fahlberg is a crook. It would make me sick if someone used my name in the same breath with his.”
References
[1] S.W. Mintz, Die süße Macht (in German), Campus Verlag, Frankfurt/New York, 1987. ISBN: 3-593-33660-X
[2] C. M. Merki, Zucker gegen Saccharin (in German), Campus Verlag, Frankfurt/New York, 1993. ISBN: 3-593-34885-3
[3] C. M. Merki, Genussmittel (in German), Campus Verlag, Frankfurt/New York, 1999. ISBN: 3-593-36337-2
[4] O. Krätz, E. Vaupel, Angew. Chem. Int. Ed. 2007, 46, 24. DOI: 10.1002/anie.200602455
[5] G. B. Kauffman, P. M. Priebe, Ambix 1978, 25, 191. DOI: 10.1179/amb.1978.25.3.191
[6] I. Remsen, Liebigs Ann. 1875, 178, 275 (in German). DOI: 10.1002/jlac.18751780212
[7] M. W. Iles, I. Remsen, Ber. Dtsch. Chem. Ges. 1878, 11, 229 (in German). DOI: 10.1002/cber.18780110150
[8] C. Fahlberg, I. Remsen, Ber. Dtsch. Chem. Ges. 1879, 12, 469. DOI: 10.1002/cber.187901201135
[9] C. Fahlberg, 25 Jahre im Dienste der Saccharin-Industrie (in German), 1903.
[10] I. Remsen, C. Fahlberg, Am. Chem. J. 1880, 1, 426. Link
[11] I. Remsen, A. G. Palmer, Am. Chem. J. 1886, 8, 223. Link
[12] I. Remsen, Ber. Dtsch. Chem. Ges. 1887, 20, 2274 (in German). DOI: 10.1002/cber.18870200235
[13] S. Tarbell, A. T. Tarbell, J. Chem. Educ. 1978, 55, 161. DOI: 10.1021/ed055p161
The article has been published in German as:
- Die Saccharin-Saga,
Klaus Roth, Erich Lück,
Chem. unserer Zeit 2011, 45, 406–423.
DOI: 10.1002/ciuz.201100574
and
- Kalorienfreie Süße aus Labor und Natur,
Klaus Roth, Erich Lück,
Chem. unserer Zeit 2012, 46, 168–192.
DOI: 10.1002/ciuz.201200587
and was translated by W. E. Russey.
The Saccharin Saga – Part 1
The invention of the first artificial sweetener and a lifetime battle for credit
The Saccharin Saga – Part 2
The early industrial production and organized smuggling of saccharin
The Saccharin Saga – Part 3
The health concerns associated with artificial sweeteners
The Saccharin Saga – Part 4
A glance back to ancient Rome, and the most hair-raising of all sweeteners
The Saccharin Saga – Part 5
What’s in your softdrink? – Introducing cyclamate
The Saccharin Saga – Part 6
Aspartame – a sweet dipeptide ester
The Saccharin Saga – Part 7
Acesulfame-K – another successful sweetening agent
The Saccharin Saga – Part 8
Thaumatin – a sweet protein with a licorice aftertaste
The Saccharin Saga – Part 9
Sucrose or Splenda turned into a low-calorie alternative to sucralose or saccharose
The Saccharin Saga – Part 10
Combining sweet cations and anions, and turning bitter compounds into sweeteners
The Saccharin Saga – Part 11
Intelligent synthetic strategies for low-calorie sweeteners
The Saccharin Saga – Part 12
Stevia plant extracts as low-calorie sweeteners
The Saccharin Saga – Part 13
Finding the best mixture of sweeteners to replicate the taste of real sugar
See all articles by Klaus Roth published in ChemistryViews Magazine








Great details.
Thank you for sharing.