An air of decadence swirled around the preferred drink of Parisian Bohemians as the 19th century ended. Following decades of a complete ban, this high-ethanol beverage has been back on the market in the EU since 1991, as wel as in many other countries.
Originally, the green fairy kissed creative people after a couple of glasses, inspiring Baudelaire, Verlaine, Wilde, Toulouse-Lautrec, and van Gogh to create masterpieces while in its thrall. We would like to discover what chemical trick the “fée verte” used and find out whether there is still hope for a kiss from the green fairy when drinking modern absinthe.
Most of the scientific community at the end of the 19th century was convinced that absinthe was dangerous to health while other alcoholic beverages were not. They firmly believed in a new disease known as absinthism. Scientific studies were undertaken to demonstrate the danger of wormwood in contrast to the harmlessness of ethanol, because researchers were convinced that the green fairy was to be found somewhere in wormwood.
In the experimental search for the green fairy, pure essential oil (wormwood or absinthe oil) obtained directly by steam distillation of the plants was used. This oil roughly corresponds to the volatile components obtained in the production of absinthe through distillation (see Absinthe Recipe in Part 1).
2.3 Experiments in the Search for the Green Fairy
Two experiments will be presented here:
I. 1879 Experiment
In a self-experiment, Carl Friedrich Bohm, a brave resident of Halle, Germany, drank five grams of absinthe oil on an empty stomach at 19:00. He reported on this in his doctoral dissertation [10,11]:
“After one hour, my breath smelled intensely ethereal and I had an odd, cooling taste in my mouth. There were no other subjective sensations. I had no burning in my stomach and my mental faculties were not affected in the least. The following morning, I was fully sober and ingested another five grams of oil. My breath had an odor for the entire day, but I noticed nothing else. … That was all. Perhaps the dose was not strong enough for me, but I was not inclined to try larger doses.”
This self-experiment showed that the oral ingestion of two five-gram doses of absinthe oil (70 mg/kg of body weight in each dose) leads to no subjective disorders. This foolish self-experiment was unwittingly repeated with twice the dose in 1997 [12].
II. 1997 Experiment
A 31-year-old man ordered wormwood oil over the internet and drank 10 mL of it at once in the belief he was drinking highly concentrated absinthe. A few hours later he was disoriented and had severe muscle cramps. At the hospital, his condition worsened, eventually leading to acute kidney failure. The patient was treated in intensive care for several days and released on the 8th day with normal clinical values.
2.4 Is the Green Fairy’s Molecular Formula C10H16O?
It was not possible for scientists at the end of the 19th century to identify the components in wormwood that were dangerous to health. Only at the turn of the century did it become possible for Otto Wallach (1847–1931; see Fig. 2) to isolate a ketone named thujone from arborvitae plants. Thujone was later determined to be a primary ingredient in several species of Artemisia, including wormwood and sage [13].
Figure 2. Otto Wallach, a German organic chemist and winner of the 1910 Nobel Prize, carried out pivotal research into structure determination and synthesis of terpenes.
Thujone is a colorless, slightly oily, compound that is immiscible with water and has a refreshing menthol-like smell. Its very unusual chemical structure was found to be the bicyclic ketone 4-methyl-1-(1-methylethyl)bicyclo[3.1.0] hexan-3-one (1) (see Fig. 3).
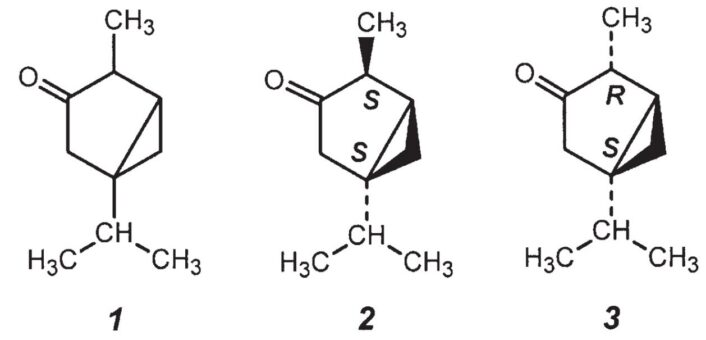
Figure 3. Thujone (4-methyl-1-(1-methylethyl)bicyclo[3.1.0] hexan-3-one) (1), α-thujone (2), and β-thujone (3).
Thujone (1) is a bicyclic monoterpene, meaning that its basic framework consists of ten carbon atoms and can formally be seen as being formed by joining together two isoprene units (2-methyl-1,2-butadiene). Thujone contains two stereogenic carbon atoms (in the 1 and 4 positions), each with four different substituents, making four stereoisomers possible. Two of these, α-thujone (2) and β-thujone (3) are present in wormwood. The two diastereomers have different chemical, physical, and toxicological properties (see Tab. 1).
Table 1. Properties of thujone.
(As is so often the case, the trivial names are confusing: there is a second iso-thujone, which is formed from the reaction of thujone with concentrated sulfuric acid. This constitutional isomer is formed by opening of the three-membered ring and is a cyclopentenone derivative.)
| Specific Rotation | Boiling Point | LD50 (mouse) | ||
|---|---|---|---|---|
| α-thujone (2) | (-)-thujone | -19.9 °C | Kp17 = 84 °C | 87.5 mg/kg |
| β-thujone (3) | (+)-isothujone [14] | +73.4 °C | Kp17 = 86 °C | 442.5 mg/kg |
The two naturally occurring isomers of thujone were not available for experiments until the first decades of the 20th century. Because of the ban, however, absinthe, wormwood, and thujone at that time were no longer of toxicological interest. The search for the green fairly was abandoned, though thujone continued to be the prime suspect.
In the next part of this article, we will examine how toxic thujone is and how much of it is absorbed when drinking – or whether absinthe is harmful.
Finally, a little excursion into the ways to enjoy absinthe:
3 The Fine Art of Drinking Absinthe
 An old postcard image stated that absinthe is not simply drunk, it is celebrated! Any connoisseur should have the proper equipment at hand: an absinthe glass and a slotted absinthe spoon.
An old postcard image stated that absinthe is not simply drunk, it is celebrated! Any connoisseur should have the proper equipment at hand: an absinthe glass and a slotted absinthe spoon.
First, 2 cL of absinthe are poured into the glass. The slotted spoon (see Fig. 4) is laid across the top of the glass, with one or two – depending on your preference – sugar cubes placed on it. At this point, one of two rituals is observed:
The Czech Ritual
The sugar cubes are sprinkled with absinthe and set on fire. When the sugar begins to bubble and caramelize, the flame is extinguished, and the spoon dipped into the absinthe in the glass. Water is then added to achieve a 1:4 to 1:5 ratio.
The French Ritual
Ice-cold water is slowly and carefully dribbled onto the sugar cubes. With a dreamy look in their eyes, the drinker watches the sugar water drip into the absinthe. At first, streaks begin to form, followed by a milky green emulsion (louche effect). The addition of water is stopped at a 1:4 or 1:5 dilution.
One sip will reveal the source of the absinthe: absinthes from France, Spain, or Switzerland are dominated by a strong note of anise, similar to (modern) pastis; Czech absinthes are distinguished by a stronger note of mint.

Figure 4. Absinthe spoon.
Absinthe Today
To enjoy the very different flavors of absinthe offered today, it is often drunk without sugar, but only with a thin stream of ice-cold water.

Figure 5. Antique absinthe glass with spoon (right) and modern absinthe glasses with two different absinthes mixed with cold water (middle). (photos taken at Absinthe House in Heidelberg, Germany)
References
[9] J. Emmert, G. Sartor, F. Sporer, J. Gummersbach, Determination of α-/β-Thujone and related terpenes in absinthe using solid phase extraction and gas chromatography, Dtsch. Lebensm. Rundschau 2004, 100(9), 352–356.
[10] Über die Wirkung des ätherischen Absinthols, Universität Halle, Germany, 1879.
[11] Helmut Werner, Absinth, Ullstein Taschenbuchverlag, München, Germany, 2002. ISBN 13: 9783548363738
[12] Steven D. Weisbord, Jeremy B. Soule, Paul L. Kimmel, Poison on Line — Acute Renal Failure Caused by Oil of Wormwood Purchased through the Internet, N. Engl. J. Med. 1997, 337, 825–827. https://doi.org/10.1056/NEJM199709183371205
[13] E. Gildemeister, F. Hoffmann, Die ätherischen Öle, Bd.III C, Akademie-Verlag, Berlin, Germany, 1963, 270.
[14] L. Novotný, V. Herout, F. Šorm, On terpenes. CIX. A contribution to the structure of absinthin and anabsinthin, Collect. Czech. Chem. Commun. 1960, 25, 1492–1499. https://doi.org/10.1135/cccc19601492
The article has been published in German as:
- Der Zauber der Grünen Fee,
Klaus Roth,
Chem. unserer Zeit 2005, 39(2), 130–136.
https://doi.org/10.1002/ciuz.200590010
and was translated by Caroll Pohl-Ferry.
Absinthe – The Magic of the Green Fairy – Part 1
When drinking modern absinthe, is there still a chance of getting a kiss from the Green Fairy like the Bohemian Parisians at the end of the 19th century?
Absinthe – The Magic of the Green Fairy – Part 3
How toxic is Thujon and how much of it is ingested when drinking Absinthe?
See similar articles by Klaus Roth published on ChemistryViews.org




