Have you ever heard of the element damarium? There was a publication about it on April 2, 1890. Gisela Boeck, Professor at the University of Rostock, Germany, and head of the History of Chemistry Division of the German Chemical Society (GDCh), told me about the publication. It is a very exciting story that proves that chemistry was already very humorous at the end of the 19th century—or that even then, there were fake articles and unreliable reviewers.
Elements Known in the 1890s
In 1889, according to the article, the German researchers K. Lauer and P. Antsch discovered a new element on a plateau in present-day Namibia. During an excursion, the mining engineer K. Lauer and his team managed to capture an unusual gas. P. Antsch examined it in a laboratory in Cape Town, South Africa, and was able to extract and analyze the new element. Both published their results on April 2, 1890.
As described in the article, the discovery of new elements was and is today a difficult, laborious, slow, and unpredictable process. In 1869, Dmitri Ivanovich Mendeleev (1834–1907) presented his first version of what later became known as the periodic system or table of the elements. At that time, there were 60 elements, and Mendeleev’s system predicted the existence of others. Mendeleev claimed that the gaps in his system corresponded to three unknown elements with atomic numbers about 44, 68, and 72, resembling their lighter analogues boron, aluminum, and silicon, respectively. When the three elements were discovered in nature between 1875 and 1886, they were named scandium (1879), gallium (1875), and germanium (1886) after the places they were discovered, and their properties were found to be very similar to those predicted by Mendeleev. By 1890, when our article appeared, 74 elements were known. The stage for discovering elements was then Europe.
Heroic Discovery
But back to damarium, for which there is no gap in the periodical system. K. Lauer describes that he together with his expedition team found 17 funnel-shaped depressions with 0.2-0.8 m large, circular openings and a depth of about 2 m on a plateau of about 2 km² as well as a peculiar smell. The soil, otherwise described as pale red due to iron oxide, was greenish-gray around the depressions. There was no vegetation, but a lot of dead, desiccated insects, birds, and larger animals, which were colored strangely. That does not sound very confidence-inspiring. However, the scientists were true heroes, curious and unafraid to take a closer look. After no more than 20 minutes, they describe, their clothes and hair also changed color. But apparently, no one was harmed, as the article was published a year after the expedition.
The researchers observed a rising plume of air from the funnels and a faint sound they described as a sound similar to a distant tea kettle or cicada chirp. Before collecting the gas in empty cognac bottles and sealing them with wax to send them to the chemist P. Antsch in South Africa, K. Lauer and his colleagues made some tests themselves, such as inhaling the gas deeply and igniting it in one of the funnels. True heroes give all to science.
Superelement
According to his report of April 1, 1889, the chemist P. Antsch found that the gas in contact with water formed hydrogen and a liquid. The liquid that collected above the water was odorless, colorless, easily movable, strongly light-breaking, and reacted neutrally. With a grain of zinc, a turbulent gas development was observed. The gas has a musk-like odor, burns with a fiery gray flame, and forms a violently exploding mixture with oxygen.
Through electrolysis, P. Antsch discovered that the liquid was an oxide of a previously unknown element. He named it damarium (D) after the Damara, the inhabitants of the site where it was found. The gas captured during the expedition is a mixture that he calls fumarole gas.
Damarium oxide has the formula D4O and D is said to be twice as light as hydrogen. P. Antsch says that damarium is “the actually inert element of smallest atomic weight, which must henceforth be the basis of our atomic weight system”. P. Antsch proposes that from now on H, Cl, … are two-valued, O, S, … four-valued, etc. And continues in this report: “Naturally, the stereochemical way of thinking, as we owe it to Le Bel and van’t Hoff, is thus given a severe blow, so that e.g. the ‘tetrahedral way of thinking’ is simply no longer possible”. At least not the way we know it.
Damarium also proves to be absolutely outstanding: It is the most powerful reduction tool imaginable. For example, P. Antsch describes the following:
- D immediately removes metal from solutions of platinum, gold, silver, lead, and copper salts.
- D immediately precipitates sulfur when passed through aqueous SO2 solution; when passed through moderately dilute sulfuric acid, shortly afterward H2S is formed.
- Ferric salts are immediately reduced to ferrous salts; calomel (Hg₂Cl₂) precipitates out of the sublimation solution.
- Indigo carmine, or 5,5′-indigodisulfonic acid sodium salt, solution is instantly discolored; nitrobenzol is converted to aniline.
- D reduces CO2 to CO.
P. Antsch reports that D4O is not soluble in alcohol or water. Other amazing properties are tested and described, such as when D4O is passed through phosphoric acid anhydride, meta-damarophosphoric acid or possibly ortho-damarophosphoric acid is formed.
The fumarol gas collected during the expedition is a mixture of D, D4O, H, CO, and probably nitrogen and possibly hydrocarbons, according to P. Antsch.
Outlook
P. Antsch’s report concludes that he is eagerly awaiting larger quantities of the gas to conduct further studies. He concludes that “damarium is a perhaps widespread, but mostly very sparingly occurring and therefore so far overlooked element”.
Well, apparently so “sparingly occurring” that it has never been reported on again. So, unfortunately, we have to continue to search for methods of CO2 conversion. However, perhaps it’s also quite fortunate that this superelement has not reappeared.
Notes
In German, the names of the expedition member K. Lauer (sounds like Kalauer; play on words) and the chemist P. Antsch (Panscher, someone who waters down something) make an amusing analogy.
In many countries, April Fools’ Day or All Fools’ Day is an annual custom on April 1 involving practical jokes and hoaxes.
Sources
- K. Lauer, P. Antsch, Ein neues gasförmiges Element, Chem.-Ztg. 1890, 14, 435-436.
- Helge Kragh, The Periodic System between Chemistry and Physics, ChemistryViews 2019. https://doi.org/10.1002/chemv.201900040
- Hans-Jürgen Quadbeck-Seeger, History of the Discovery of the Elements, ChemistryViews 2010. https://doi.org/10.1002/chemv.201000006
Also of Interest
April Fools’ Day

Fridolin E. Coli, gram-negative rod-shaped bacterium, talks about his more than one hundred years of experience in research

Interview with Erwin Reicher, first ever Professor of Applied Popularity Research, University of Kleinzack, on how to make chemistry popular
New Elements

The synthesis of heavy elements
C. E. Düllmann, GSI Darmstadt, gives insight into the chemistry of short-lived, superheavy atoms
M. Block, GSI Darmstadt, gives an insight into the physics of short-lived, superheavy atoms

The chronology of the discovery of the elements is a textbook example of scientific development
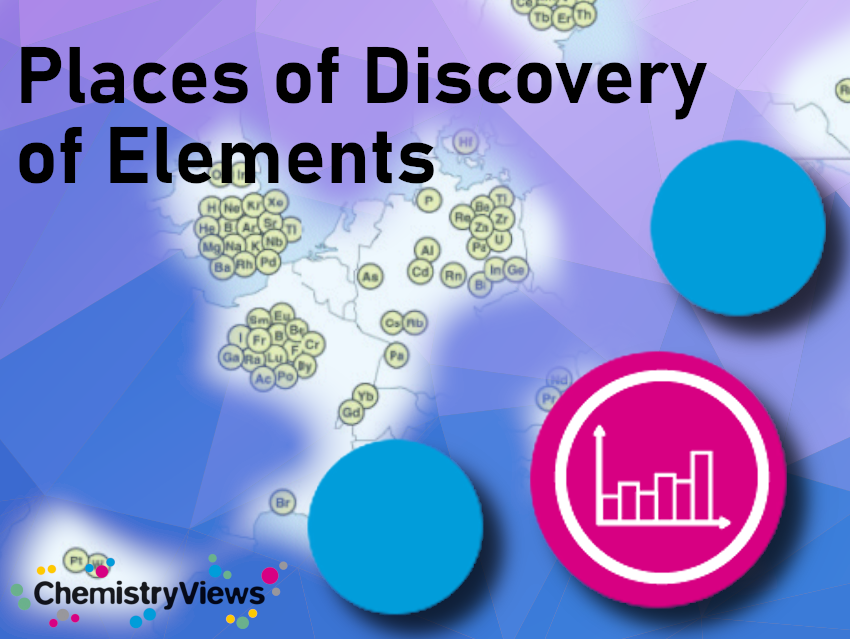
All 92 naturally occurring elements were identified in Europe while the artificial elements were mostly discovered in the USA

In the 1920s the periodic system turned out to be a macroscopic representation of the internal structure of the atoms – making it as much about physics as it is about chemistry




