The periodic table of the elements is one of mankind’s greatest discoveries in nature since it encompasses all the building blocks that bind our universe together at its heart: from the tiniest virus to the most distant galaxy.
1. The Transuranic Elements
With the discovery of promethium in 1947 [1], the periodic system of the elements (PSE) was regarded as complete, from hydrogen (atomic number Z = 1) to uranium (Z = 92). However, it was already known at that time that uranium did not really represent an upper limit. As early as 1940 the first transuranic element, element 93 (neptunium), had already been prepared—by neutron bombardment of uranium [2]. From this starting point, it has proven possible in the course of the last 75 years to expand the PSE by 25 artificial elements. Preparation of heavy actinides and transactinides has been based on four distinct synthetic strategies [3–6] (see Tab. 1):
- Elements 93–101: stepwise bombardment of uranium (Z = 92) with neutrons and very light projectile nuclei (Z = 1–2),
- Elements 102–106: bombardment of elements 94–98 with light projectile nuclei (Z = 5–10),
- Elements 107–112: bombardment of elements 82 and 83 (lead, bismuth) with moderately heavy projectile nuclei (Z = 34–40).
- Elements 113–118: bombardment of elements Z = 94–98 with Ca-48 projectile nuclei (Z = 20).
|
Table 1. Synthesis and stability of heavy atomic nuclei [7]. Reported half-lives refer to the longest-lived isotope. |
.jpg) |
The stability of atomic nuclei decreases from thorium (Z = 90) to element 118 by more than 21 (!) powers of ten (see Tab. 1). This poses the urgent question of whether atomic nuclei above a certain atomic number will prove so unstable that they can no longer be produced. In order to help find out what it is that causes nuclear scientists to attempt ever more difficult nuclear syntheses, let us accompany some of them in their efforts to make element 118, the heaviest one known so far.
2. The Preparation of Transactinides
2.1. Nuclear Fusion
To accomplish a nuclear fusion, the lighter atom is first ionized to the point of bearing multiple positive charges, then accelerated in an electric field to 10 % the speed of light, and fired as a projectile at the second element, which serves as a target. The probability that the two nuclei will fuse is vanishingly small, however, and the heavier the desired fusion nucleus, the more improbable the event.
With the instrumental options available today, and for the very heavy transactinides, one achieves perhaps a single fusion event in a day, often only one in a week, a month, or a year [8]. Truly, and no joke: possibly just one atom per year!
Worse yet, if in the course of the bombardment, at some time or other, one transactinide atom does form: it will prove to be unstable, and decay within a few milliseconds. This means that creation and detection of a single (!) atom must occur virtually simultaneously. What a challenge!
2.2. Theoretical Example: Element 118
We begin by going through, on paper, the example of element 118 (see Fig. 1). Consider shooting calcium ions (Z = 20) as projectiles at californium (Z = 98) as a target, at a rate of 1012 to 1013 times per second. In the case of a successful fusion (say, once every 1016 to 1017 collisions), what forms initially is a so-called “compound” nucleus. Its make-up represents the sum of neutron and proton counts for both of the starting nuclei: in this case 118 protons and 179 neutrons. Because of the high energy of the accelerated Ca projectiles, any compound nucleus, after its formation, will still be very energy-rich and will stabilize itself within 10–12 seconds by eliminating a few (in this case three) neutrons. Through this so-called “evaporation” process, we will have achieved our goal: the creation of an atom of 294[118] through fusion.
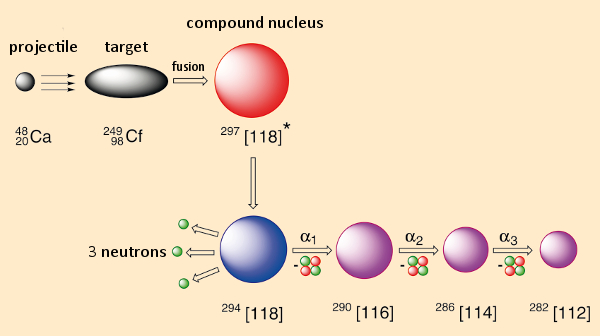 |
|
Figure 1. Synthesis of element 118. |
Our triumph will go unnoticed, however, since this single fusion-nucleus 118 itself will not be detectable. Its detection can only be achieved indirectly, in what could constitute the basis of a Greek tragedy: only through its decay does such a fusion nucleus leave traces from which we can conclude that it once existed. Decomposition here entails release of α-radiation (a 4He nucleus), an event for which the location and time of origin, as well as the associated energy, can, in fact, be measured relatively accurately. But the presence of the nucleus of element 116, which results from element 118 eliminating an α-particle, also cannot be demonstrated directly. Its existence and lifetime can again be detected only on the basis of decay products. By the way: With the radioactive decay of a single atom of this sort it becomes possible to report only its lifetime; the more characteristic “half-life” can be established only through a large number of decay events.
With such a decay series, a typical feature for a transactinide, we become acquainted with an entire family of nuclei, including individual lifetimes and energies for the decay products — but without ever directly observing a single family member.
When one is spared the frequent problem of spontaneous nuclear decay, it may even be possible to pursue a decay chain all the way to an already familiar nucleus. Then one can trace backward the atomic masses of all members of the decay sequence and thereby assure with certainty the existence of the starting nucleus.
2.3. Instrumental Challenges
Before we examine in greater detail the actual preparation of element 118, we should consider the immense instrumental demands imposed by the entire experiment. In the course of bombarding the target with highly accelerated ions, a number of side reactions can also take place. Every second, roughly a billion (!) unwanted decay products accumulate [9], whereas a desired fusion nucleus arises perhaps once a day—or indeed even less frequently.
For this reason, an enormous technical effort is required to sort out that which is sought from the utterly uninteresting [10–14]. Despite effective physical separation methods, there always remains at the end a great decay jumble, from which the extremely rare temporal, spatial, and energetically coherent α-decay sequences must be sought out. This enormous sorting process can be mastered only with the aid of highly intelligent software.
References
[1] J. A. Marinsky et al., The Chemical Identification of Radioisotopes of Neodymium and of Element 61, J. Am. Chem. Soc. 1947, 69, 2781–2785. https://doi.org/10.1021/ja01203a059
[2] E. McMillan, P. H. Abelson, Radioactive Element 93, Phys. Rev. 1940, 57, 1185. https://doi.org/10.1103/PhysRev.57.1185.2
[3] C. Keller, Die Künstlichen Elemente (in German), Chem. Unserer Zeit 1967, 1, 167–177. https://doi.org/10.1002/ciuz.19670010602
[4] C. Keller, Transurane: Anwendungen und chemische Eigenschaften (in German), Chem. Unserer Zeit 1972, 6, 75–82. https://doi.org/10.1002/ciuz.19720060303
[5] J. V. Kratz, Chemie der schwersten Elemente (in German), Chem. Unserer Zeit 1995, 29, 194–206. https://doi.org/10.1002/ciuz.19950290406
[6] M. Schädel, Chemistry of the superheavy elements, Phil. Trans. Royal Soc. A 2015, 373, 20140191. https://doi.org/10.1098/rsta.2014.0191
[7] National Nuclear Data Center, Brookhaven National Laboratory, Nuclear Wallet Cards Search, www.nndc.bnl.gov.
[8] M. Schädel, Chemie superschwerer Elemente (in German), Angew. Chem. 2006, 118, 378–414. https://doi.org/10.1002/ange.200461072
[9] Y. T. Oganessian et al., The synthesis of element 114 confirmed decades-old theoretical predictions of a little patch of nuclear stability in a sea of short-lived superheavy nuclei, wwwnew.jinr.ru.
[10] GSI Helmholtzzentrum für Schwerionenforschung, www.gsi.de.
[11] GSI Helmholtzzentrum für Schwerionenforschung, Erzeugung neuer Elemente – die Idee (in German), www.gsi.de
[12] RIKEN Nishina Center for Accelerator-Based Science, www.nishina.riken.jp.
[13] Discovery of Transuranium Elements at Berkeley Lab, www.acs.org.
[14] Joint Institute for Nuclear Research, www.jinr.ru.
The article has been published in German as:
- Ist das Element 118 ein Edelgas?,
Klaus Roth,
Chem. unserer Zeit 2017, 51, 418–426.
https://doi.org/10.1002/ciuz.201700838
and was translated by W. E. Russey.
New Kids on the Table: Is Element 118 a Noble Gas? – Part 1
The synthesis of heavy elements
New Kids on the Table: Is Element 118 a Noble Gas? – Part 2
The difficult road towards element 118
New Kids on the Table: Is Element 118 a Noble Gas? – Part 3
The first synthesis of element 118, its properties, and naming new elements
See similar articles by Klaus Roth published in ChemisteyViews Magazine




