The periodic table of the elements is one of mankind’s greatest discoveries in nature since it encompasses all the building blocks that bind our universe together at its heart: from the tiniest virus to the most distant galaxy. Last time, we discussed the synthesis of heavy elements. In this part, we have a look at the difficult road towards element 118.
3. The First Approach to Element 118
On the strength of our purely theoretical “paper synthesis”, we proceed to consider the first real attempt to prepare element 118 (recently designated officially as Og: oganesson).
Robert Smolańczuk
The story begins with a publication by the Polish nuclear physicist Robert Smolańczuk, who in 1999 estimated the success prospects for various possible synthetic approaches to element 118 on the basis of model calculations. In the process, he went unusually far out on a limb for a theoretician [15,16]: “… we came to the conclusion that the most promising synthesis of element 118 would entail bombardment of a lead-208 target with krypton-86 projectiles.”
Albert Ghiorso
This idea was immediately seized upon at the Lawrence Livermore National Laboratory (LLNL) in Berkeley, CA, USA, where all the necessary isotopes and instrumental facilities were readily available. A member of the research group there, Albert Ghiorso, who had already been involved in the discovery of numerous transuranic elements, observed in retrospect:
“Smolańczuk suggested this peculiar reaction, which no one believed would succeed. But since it should be relatively easy to carry out, we thought: what the heck, we have nothing to lose by trying it!”
Victor Ninov
A research group at LLNL under the direction of Kenneth Gregorich started carrying out the proposed experiment within a few weeks. Over the course of five days, a lead target was bombarded with 19-fold-positive krypton ions. A computer-supported evaluation of the massive set of acquired data was undertaken by Dr. Victor Ninov. This Bulgarian physicist had earned his doctorate at the Technical University (TU) Darmstadt, Germany, and had already participated successfully in the discovery of elements 110–112 at the Society for Heavy Ion Research (Gesellschaft für Schwerionenforschung, GSI), also in Darmstadt. He was, in fact, regarded as a leading expert in this field.
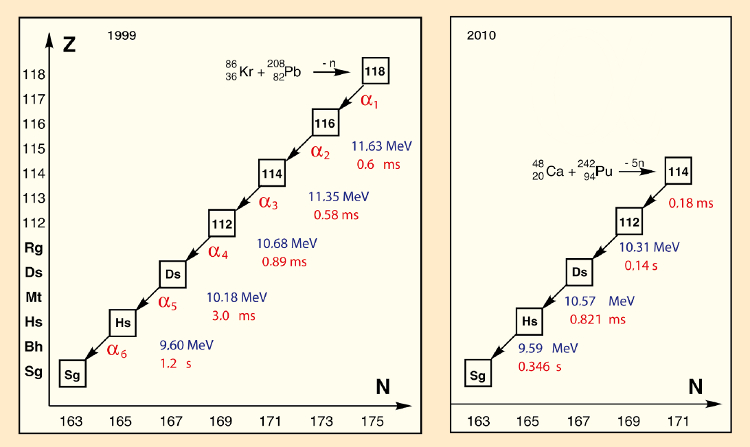
Ninov was able to identify here three α-decay series beginning with element 118, proceeding by way of the still unknown element 116, to give seaborgium (Sg, Z = 106; see Fig. 2). Albert Ghiorso remarked “It was such a surprise that, at first, we ourselves could not believe it. Robert Smolańczuk’s predictions seemed to be entirely confirmed.” The results were published with Dr. Ninov as principal author and 14 additional coauthors [17]. It represented a tremendous success for the LLNL.
 |
|
Figure 2. In 2010, it became possible to replace a significant part of the counterfeit decay series (left) with information characterized experimentally in an independent way (right) [18]. A comparison shows that the falsification was devised quite intelligently. |
4. Doubts are Raised
Before a newly discovered element can be added to the Mount Olympus of the periodic table, its existence must first be confirmed by independent laboratories. Corresponding control experiments for preparation of 118 at the GSI in Darmstadt and RIKEN in Japan failed, however. Still, various technical explanations were possible, so the Berkeley group itself set out to attempt a repeat in the spring of 2001.
Things looked bleak at first, because the researchers were unable to find a decay sequence attributable to element 118. Toward the end of the experiment, however, Victor Ninov did indeed succeed, locating one of the expected decay series within the raw data.
In the meantime, another coworker, Dr. Walter Loveland, had become intimately familiar with the analytical software, and, for safety’s sake, he reexamined the raw data. To the great surprise of all, the decay series reported by Dr. Ninov was nowhere to be found! To clarify the situation, an internal investigative commission was deployed, but they were also unable to find the missing decay series.
After several similar failed attempts, by multiple additional investigative commissions, the conclusion was finally reached that there was “clear and convincing evidence” that Dr. Victor Ninov had somehow contrived the results. He was dismissed in May, 2001. Dr. Ninov denied all the charges leveled against him, but was unable to explain the inconsistencies in the data. He pointed out, however, that changes in the raw data could have been introduced by anyone in the laboratory, since his password was an open secret within the research group.
On July 15, 2001, the discovery of element 118 was formally withdrawn in Physical Review Letters [19]. The recall began with the sentence: “All but one of the authors of the original Letter have asked us to publish the following retraction: …” None of the scientists involved came away unscathed. The final investigative commission wrote in its report: “The committee finds it incredible that not a single collaborator checked the validity of Ninov’s conclusions of having found three element 118 decay chains by tracing the events back to the raw data tapes” [20].
References
[15] R. Smolańczuk, Production mechanism of superheavy nuclei in cold fusion reactions, Phys. Rev. C 1999, 59, 2634. https://doi.org/10.1103/PhysRevC.59.2634
[16] R. Smolańczuk, Production of superheavy elements, Phys. Rev. C 1999, 60, 021301. https://doi.org/10.1103/PhysRevC.60.021301
[17] V. Ninov et al., Observation of Superheavy Nuclei Produced in the Reaction of 86Kr with 208Pb, Phys. Rev. Lett. 1999, 83, 1104. https://doi.org/10.1103/PhysRevLett.83.1104
[18] P. A. Ellison et al., New Superheavy Element Isotopes: 242Pu(48Ca,5n)285114, Phys. Rev. Lett. 2010, 105, 182701. https://doi.org/10.1103/PhysRevLett.105.182701
[19] V. Ninov et al., Editorial Note: Observation of Superheavy Nuclei Produced in the Reaction of 86Kr with 208Pb [Phys. Rev. Lett. 83, 1104 (1999)], Phys. Rev. Lett. 2002, 89, 039901. https://doi.org/10.1103/PhysRevLett.89.039901
[20] G. Johnson, At Lawrence Berkeley, Physicists Say a Colleague Took Them for a Ride, The New York Times, October 15, 2002.
The article has been published in German as:
- Ist das Element 118 ein Edelgas?,
Klaus Roth,
Chem. unserer Zeit 2017, 51, 418–426.
https://doi.org/10.1002/ciuz.201700838
and was translated by W. E. Russey.
New Kids on the Table: Is Element 118 a Noble Gas? – Part 1
The synthesis of heavy elements
New Kids on the Table: Is Element 118 a Noble Gas? – Part 2
The difficult road towards element 118
New Kids on the Table: Is Element 118 a Noble Gas? – Part 3
The first synthesis of element 118, its properties, and naming new elements
See similar articles by Klaus Roth published in ChemistryViews Magazine




