To produce the rainbow of colors on today’s make up counters, chemists derive dyes and pigments from a variety of compounds. We list a selection below.
Black – Fe3+[Fe3+Fe2+]O4
This crystallizes in an inverse spinell structure. Electrons can exchange quickly within the mixed valent iron center in the octahedral spaces.
Brown – γ-Fe(III)-oxid and α-Fe(III)-oxidhydroxid
Green – Cr(III)-oxid
The oxygens surrounding the ocathedral Cr split up the otherwise degenerate, partly occupied d orbitals of the cation. This crystal field splitting allows d–d-crossover, the activation energy of which is provided by photons of visible light.
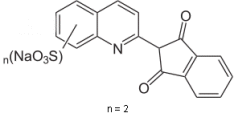
Yellow – Chinolin Yellow Lack
The crosswise delocalization of the π-system over the three heterofunctionalities is the basis of this chromophore. It is soluble in water. By so-called “laking” (precipitation with an inert binder), it is transferred into mostly insoluble calcium, barium, iron, or aluminium salts.
Red – Lithol Rubin BK
Synthetic azo-color
.gif)
Blue – Phthalocyanin
This planar macrocyclic compound contains a π-system delocalised over the whole molecule. Its HOMO-LUMO transition has an absorption maximum in the yellow/orange range of the visible spectrum of light.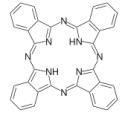
- März – Alaaf und Helau
Deike Hatscher, Sandra Knirsch,
Chem. Unserer Zeit 2011, 45 (1), 65.
DOI: 10.1002/ciuz.201190011


