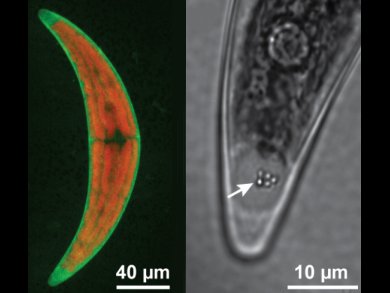
The development of safe and highly selective methods for removing 90Sr, with a half-life of approx. 30 years, from nuclear waste and the environment is critical for sustainable use of nuclear power. The chemical similarity of calcium, strontium, and barium makes this a very challenging problem for synthetic ion exchange materials and phytoremediation systems alike.
Derk Joester, Northwestern University, Evanston, IL, USA, and colleagues show that the desmid green algae Closterium moniliferum separates Ca2+, Sr2+, and Ba2+ during biomineralization. Using synchrotron X-ray fluorescence (SXRF) microscopy, they were for the first time able to quantify the composition of BaSO4 crystals mineralized in the terminal vacuoles of the desmid. Sr sequestration occurs via co-precipitation of Sr with barite.
The researchers were able to manipulate medium conditions to increase the Sr content of crystals deposited by desmids by more than two orders of magnitude, to a maximum of 45 mol%. At the same time very little calcium is incorporated into the crystal.
Permanent removal of Sr from solution could be ensured simply by harvesting cells after crystal precipitation and isolating crystals by filtration and/or ashing.
The unusual ability of desmids to selectively sequester Sr into an inert inorganic form, combined with the potential for engineering the system to increase Sr incorporation, may offer a unique alternative to traditional methods for 90Sr remediation. The implications of this work range from direct bioengineering of desmids for waste or environmental clean up, to bio-inspired materials for separation technology.
Image (C) Wiley-VCH
- Selective sequestration of strontium in desmid green algae by biogenic co-precipitation with barite,
Minna R. Krejci, Lydia Finney, Stefan Vogt, Derk Joester
ChemSusChem 2011.
DOI: 10.1002/cssc.201000448 - Presented at the 241st ACS National Meeting in Anaheim, CA, USA
Other articles related to the ACS meeting
- Hollywood Chemistry
And the Winner for the Most Accurate Science in a Movie or TV series goes to … - Kevlar-like Plastic from Pineapples and Bananas
Nano-cellulose fibers from plants may lead to stronger, lighter, and more sustainable materials to replace automotive plastics - Charles Lathrop Parson Award
Michael E. Strem, president of high purity chemicals maker Strem Chemicals, receives the ACS award for outstanding public service - Priestley Medal Awarded
Ahmed H. Zewail, California Institute of Technology, USA, awarded ACS’s highest honor Priestley Medal Awarded - Dendrimersomes: A New Mode of Drug Delivery?
Amphiphilic branched nanostructures self-assemble in water to form packets capable of mimicking biological membranes - A New Class of Therapeutics
Staple peptides are able to address targets previously considered undruggable by conventional therapeutics