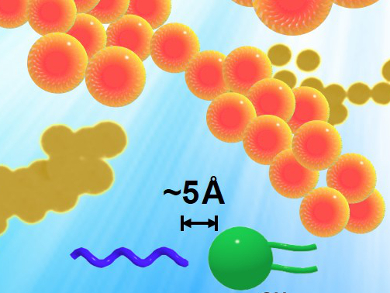
Emulsions are droplets of fluid within another immiscible fluid that are stabilized by molecules called emulsifiers. Multiple interactions are involved in stabilizing emulsions. Polymer-based emulsifiers stabilize emulsions using steric repulsions. Attractive interactions can also stabilize emulsions, and this approach can also change the fluid properties of such emulsions.
Alberto Fernandez-Nieves, Georgia Institute of Technology, Atlanta, USA, Youngbok Lee and Jin Woong Kim, Hanyang University, Ansan, Republic of Korea, and colleagues formed nanoemulsions based on the amphiphilic block copolymer poly(ethylene oxide)-block-poly(ε-caprolactone) (PEO-b-PCL) and lecithin.
The addition of lecithin stabilizes the nanoemulsions by inducing a tighter and denser molecular assembly, as confirmed by NMR spectroscopy and differential scanning calorimetry. This stability is attributed to the dipole–dipole interactions between the positively charged choline groups of lecithin and the methoxy group of PEO – and thus, the products are considered as “attractive emulsions”.
Aside from stability, the team showed that these nanoemulsions exhibit gel-like rheological properties and excellent transdermal delivery performance. This makies these nanoemulsions ideal for applications in drug delivery, cosmetic and food formulations, as well as in the life sciences.
- Structurally Stable Attractive Nanoscale Emulsions with Dipole-Dipole Interaction-Driven Interdrop Percolation,
Kyounghee Shin, Gyeonghyeon Gong, Jonas Cuadrado, Serim Jeon, Mintae Seo, Hong Sung Choi, Jae Sung Hwang, Youngbok Lee, Alberto Fernandez-Nieves, Jin Woong Kim,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201604722