High-Performance Sodium-Ion Batteries
Sodium-ion batteries (SIB) are promising candidates for a cheap and sustainable battery technology, however, a recurring issue is anode instability. Guangda Li, Qilu University of Technology, Jinan, China, and colleagues have prepared a submicron-size structured anode composite material that can accommodate large volume changes. The antimony sulfide electrode is easily prepared and exhibits superior capacity and cycling performance.
In contrast to lithium-ion batteries (LIBs), sodium-ion batteries rely on readily available and sustainable raw materials. One of the main reasons why SIBs are not yet widely used is instability: The large sodium ion cannot integrate as easily in the electrodes as the small lithium ion, causing significant expansion and shrinkage of the structures during the discharging/charging cycles. This problem particularly appears in the anode, which can simply pulverize during longer cycling periods. A working sodium ion battery can only be developed if this issue is solved.
Flowerlike Nanostructures
The researchers have combined micro- and nanostructured materials with state-of-the-art battery chemistry. They assembled an anode composite material that, through its flower-like submicrostructure, can mitigate the drastic volume changes while still showing improved conductivity and capacity. Moreover, it can be easily prepared.
Antimony, or, even better, antimony sulfide, are attractive anode materials for SIBs. Their very high theoretical specific capacities result from the fact that as much as three sodium atoms per antimony can be incorporated in the structure upon discharging (which in battery terms is sodiation). During this process, the antimony sulfide first forms sodium sulfide and then antimony alloys.
To reduce the effects of the large volume changes, microstructuring to a size between nano- and bulk materials has been proposed. The scientists prepared spherical particles of antimony sulfide between two to three microns in diameter. A closer look revealed that the surface was composed of numerous thin sheets grown together to construct aflowerlike structure. This “bunch of flowers” might serve as an effective buffer against volume changes, but its conductivity and diffusion paths are still too low for battery applications.
Antimony Sulfide–Polypyrrole Composites
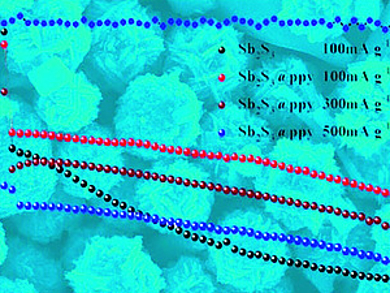
To solve this problem, the researchers coated the flowerlike structure with a layer made of polypyrrole polymer (PPy). “The PPy coating layers not only serve as the structural stabilizer […], but can also enhance the conductivity of antimony sulfide submicrospheres,” they explain. The final composite material has a well-defined shape and meets the technical demands of a high-performance anode.
The team also emphasizes that their preparation method is a straightforward sol–gel technology starting from antimony acetate (which leaves no harmful chloride in the end product), in combination with a smoothly proceeding polymerization/coating step. This work shows that the combination of nanoengineering strategies with battery electrochemistry may lead to products that can complement or substitute current lithium-ion technology.
- Flowerlike Sb2S3/PPy Microspheres Used as Anode Material for High-Performance Sodium-Ion Batteries,
Tian Zheng, Guangda Li, Lingxue Zhao, Yanxin Shen,
Eur. J. Inorg. Chem. 2018, 2018, 1224–1228.
https://doi.org/10.1002/ejic.201701364