Dried, pulverized flower heads of two chrysanthemum species (also known as “pyrethrum”) contain insecticides with nearly perfect toxicity profiles: highly toxic with respect to insects, but non-toxic to mammals. The chemical structures of these so-called pyrethrins were, for the time of their first investigation, very complex, and decades of intensive research efforts were required before derivatives (pyrethroids) could be prepared that were more readily accessible and stable, but still effective.
5. Isolation of the Active Ingredients from Pyrethrum
We turn now to the scientific content of the famous 10-part series of publications by Staudinger and Ružička [5] (see Part 1). First, the isolation of the active ingredients from pulverized chrysanthemum flower heads is described. Should you be inclined to try this at home, do as Staudinger and Ružička did, and start with 100 kg of finely ground plant material which you exhaustively extract with low-boiling petroleum ether. “Exhaustively” in this context means that you must continuously test to see whether effective insecticidal material is still being extracted.
This is in no sense trivial since these components are not colored, nor do they have any smell. Proof of their presence is possible only via their biological impact. It is recommended that one adopt the same test system (a bioassay) as that developed by Staudinger and Ružička. For this purpose, you will need to establish a small breeding stock of cockroaches—the German cockroach (Blattella germanica) has been shown to be especially reliable. Note, however, that these insects are nocturnal, and though they cannot fly, they are very agile, with speeds up to 30 cm/s. Allegedly, descendants of Ružička’s cockroaches continue to breed in the old ETH (Eidgenössische Technische Hochschule or Swiss Federal Institute of Technology) chemistry building at Universitätstraße 6 in Zurich, Switzerland [14].
After the extraction of 100 kg of pyrethrum powder and the evaporation of the petroleum ether extractant, you should have ca. 5 kg of a thick brownish-black liquid in hand that you will preferably work up further by Ružička’s method. But be careful! At every stage of the many purification and separation steps, you must verify with a few cockroaches that you are, in fact, still working with the insecticide-containing fraction. For this purpose, mix the material to be tested with flour at a dilution of 1:500, use the mixture to powder a dozen cockroaches, and keep an eye on their wellbeing. If the flour preparation contains insecticide, within a few minutes “the specimens will begin to show listlessness or sluggishness; some will lose the ability to run, and after about an hour, they will die” [13].
6. Chemical Structures of the Pyrethrins, According to Staudinger and Ružička
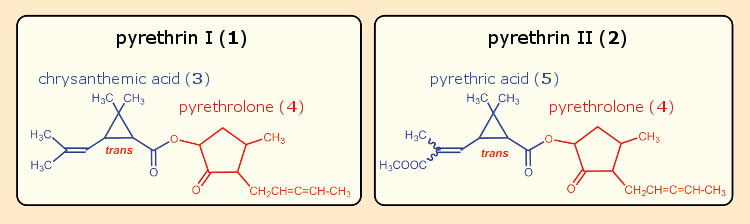
Staudinger and Ružička were ultimately able to isolate two active ingredients from pyrethrum, which they designated pyrethrin I (1) and II (2). On the basis of chemical degradation reactions, they proposed structures for both of the pyrethrins (see Fig. 6). The alcohol components of the two esters were identical, pyrethrolone (4), but they differed in their carboxylic acid portions. In the case of pyrethrin I (1), the acidic component is chrysanthemic acid (3), and in pyrethrin II (2), it is the rather similar pyrethric acid (5). It was necessary to correct a few details in the pyrethrolone structure later for both the five-membered ring and the allene side-chain unit [15].
 |
|
Figure 6. Pyrethrins I and II according to Staudinger & Ružička. |
Staudinger and Ružička wanted to use these structural formulas as “synthetic springboards” in order to access insecticides that might even be more effective. Thus, after a saponification of pyrethrin I, they began working with the resulting chrysanthemic acid (3) and pyrethrolone (4), again esterifying them, this time with other alcohols and carboxylic acids, respectively. They eventually prepared a total of 82 new esters, and also developed an easy synthesis of racemic chrysanthemic acid.
Despite numerous efforts, they were unable to synthesize pyrethrolone (4), which proved accessible to them only via cleavage of the natural products. So one of the goals, synthesis of the active ingredients pyrethrin I or II, was not achieved. In his “Arbeitserinnerungen” (“Work Reminiscences”) Staudinger revealed his great disappointment in this respect [10]: “Since a synthesis of the pyrethrins themselves was not very promising, an attempt was made to see if esters of chrysanthemic acid with other alcohols, especially terpene alcohols, would show activity, and furthermore, if it would not be possible to acquire active substances by esterification of pyrethrolone with other acids. Every effort failed to produce success.”
7. Insecticides between the two World Wars
After this disappointment, it was understandable that neither Staudinger nor Ružička ever again worked with pyrethrum. They were forced to accept the fact that, with the chemical methods of their time, it was impossible to improve upon these agents. Nevertheless, their efforts had attracted the interest of the chemical industry. Firms like Merck in Darmstadt, Germany, and Riedel-de-Haën in Berlin, Germany, which had a great deal of experience in working-up natural products, included the preparation of pyrethrum concentrates in their manufacturing programs.
Dried, ground flower heads, with a pyrethrin content of ca. 1.3 %, were extracted with hexane or kerosene and processed to a dark raw extract containing ca. 30 % pyrethrins. In contrast to Zacherl (see Part 1), these companies invested considerable effort in the development of analytical test methods that permitted them to acquire reliable data regarding the active-ingredient content of their preparations. In the form of aqueous emulsions or solutions in petroleum ether, these were then sold to insecticide producers, who, in turn, finished them as commercial products.
7.1. Germany
The popularity of pyrethrum in Germany at the time was a consequence of its low human toxicity. A few other natural products used in plant protection shared this characteristic, including quassia wood and derris root. But these substances also had the disadvantage that they had to be imported from abroad: quassia wood from the West Indies and Jamaica, and derris root from East Asia.
There were, indeed, hopes for a long-term stable supply of pyrethrum from Dalmatia and Montenegro, but traumatic experiences during the First World War had not been forgotten, when the Germans had become bitterly aware of their heavy economic dependence on foreign raw materials. Therefore, after the war, they tried to achieve the most extensive economic self-sufficiency possible with all available means, and to prepare insecticides from domestic raw materials, not ones that depended on imports.
For the manufacture of calcium arsenate, which in those days was frequently used as a pesticide, the German Empire was entirely self-sufficient. The arsenic required in the synthesis came from Silesia, the Erzgebirge, or the Harz. With respect to the preparation of another often employed pesticide, 4,6-dinitro-o-cresol, Germany was again spared from imported raw materials: the cresol was derived from German coal tar, and the nitric acid required for its nitration was prepared using the Haber-Bosch process and the Ostwald method, both developed domestically [16].
One drawback of both calcium arsenate and dinitrocresol, however, was that they represented substantial health risks for vintners and forest managers. It was thought, though, that these could be minimized by adherence to relevant protective and precautionary measures.
Another step in the direction of self-reliance on the part of the German chemical industry in the years after the First World War was the development of insecticides on the basis of chlorine-containing compounds. Fritz Haber, in particular, championed engaging the chlorine industry. This industry had been significantly expanded during the war and, given the conditions now applicable under the peace treaty, the sector might reasonably be repositioned from the production of chemical weapons to a future production of non-military pesticides [17].
7.2. Great Britain
Economically dubious dependencies on pyrethrum imports had been established not only in Germany. In order to become independent of foreign suppliers, Great Britain undertook the cultivation of pyrethrum in its crown colony Kenya in 1928, which offered ideal preconditions for the purpose [18,19]. The investments and developmental efforts paid off, since up into the 1960s, household insecticides produced in Great Britain were based predominantly on Kenyan pyrethrum.
7.3. USA
The United States were desperately in need of an insecticidal substitute during the First World War—because the war led to a loss of pyrethrum deliveries from Dalmatia. At that time, the U.S. purchased more than 80 % (!) of the total Japanese harvest of pyrethrum [20]. Even after the war ended, the U.S. demand for pyrethrum remained high, not least because—thanks to the development of easily employed hand-spraying devices—private households also became major consumers of insecticides.
An attempt to cover the growing domestic demand for pyrethrum by cultivating it in the United States itself proved a failure. For one thing, because the climatic conditions were unsuitable, leading to a low and highly variable pyrethrin content, and also because of the high costs associated with labor-intensive cultivation [21]. An enormous pyrethrum demand for combatting malaria in the U.S. south and increasing political tensions in Asia caused the United States to institute its own extensive research and development program in the 1930s, with the goal of seeking more effective pyrethrin use—and in the long term, pyrethrin substitutes.
As noted above, after the First World War, Germany turned to providing insecticides based on heavy metal compounds and coal-tar derivatives, so the research undertaken by Staudinger and Ružička was not pursued by any German research groups. Pyrethrum research moved from Germany to Japan (Sumitomo Chemical Co.), Great Britain (Rothamsted, Harpenden, UK), and the United States (U.S. Department of Agriculture).
8. In Search of a More Potent Pyrethrum: The Synergists
8.1 MYL-Powder
In the context of state-sponsored research in Great Britain, and especially the United States, thousands and thousands of substances were tested for insecticidal properties. Between 1938 and 1941 alone, 75 relevant US patents were issued, in which 1,400 synthetic and natural substances were suggested as pyrethrin substitutes or supplements.
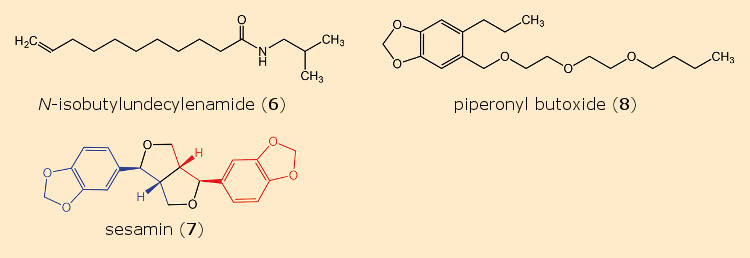
Dr. Alfred Weed of the firm John Powell & Co., New York City, USA, investigated a host of compounds over a period of four years, both alone and in combination with pyrethrins. Among these was N-isobutylundecylenamide (6) (see Fig. 7), which itself showed only weak insecticidal characteristics, but as a supplement to pyrethrins, multiplied their lethal effects: 40 mg of pyrethrins, upon addition of 420 mg of amide 6, proved more toxic to flies than 100 mg of pyrethrin alone.
 |
|
Figure 7. Pyrethrin synergists. |
This meant that the use of expensive pyrethrins in insecticides could be cut in half. Such a mixture was the basis of the so-called MYL-Powder, which the American military used in large quantities after September 1942, especially against clothes lice. The powder itself consisted of: 0.2 % pyrethrins (from a 20 % concentrate), 2 % N-isobutylundecylenamide, 0.25 % phenol S (isopropyl cresol) as an antioxidant, 2 % 2,4-dinitroanisol for killing insect eggs, and the sheet silicate pyrophyllite as a matrix material.
8.2. Piperonyl Butoxide
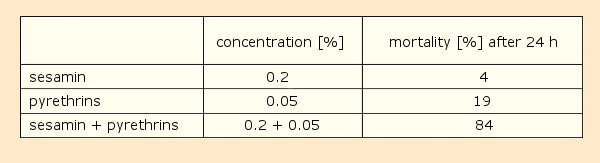
Another intriguing discovery was described by Craig Eagleson in a 1940 patent [22–25]. He added small amounts of 40 different plant- and fish oils to pyrethrin solutions, achieving the greatest synergistic effect with sesame seed oil. The origin of this remarkable impact was the constituent sesamin (7) [26, 27] (see Tab. 1).
|
Table 1. Sesamin’s influence on the toxicity of pyrethrin with respect to houseflies (solvent: kerosene with 10 % acetone; three tests with 150 flies each ). |
 |
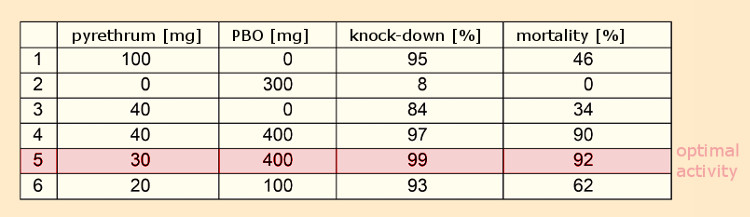
Given that, for reasons of cost, sesamin (7) itself failed to find commercial applications, a search was undertaken for structurally similar molecules. Dr. Herman Wachs of the firm Dodge & Olcott in Bayonne, New Jersey, USA, found an outstanding synergist in piperonyl butoxide, PBO (8) (IUPAC: 2‐(2‐butoxyethoxy)ethyl‐6‐propyl‐piperonylether) (see Tab. 2) [28, 29].
This compound was neither acutely toxic (LD50 (rodents) = 2.5–11.5 (!) g/kg body weight) nor mutagenic or toxic for reproduction, and continues to be added to pyrethrin preparations today. The LD50‐value is a measure of acute toxicity and corresponds to a single dose through which 50 % of the investigated animals die. PBO is, according to EU Guideline 91/414/EWG, not an active ingredient, but a “co-formulant”, permissible even in conjunction with organic agriculture.
|
Table 2. Piperonyl butoxide (PBO) as a synergist [30]. |
 |
References
[15] A. Hoffmann-Röder, N. Krause, Synthesis and Properties of Allenic Natural Products and Pharmaceuticals, Angew. Chem. Int. Ed. 2004, 116, 1196 –1216. https://doi.org/10.1002/anie.200300628
[16] E. Vaupel, Vom Teerfarbstoff zum Insektizid (in German), Chem. unserer Zeit 2012, 46, 388–400. https://doi.org/10.1002/ciuz.201200602
[17] S. Jansen, Chemical-warfare techniques for insect control: insect ‘pests’ in Germany before and after World War I, Endeavour 2000, 24, 28–33. https://doi.org/10.1016/S0160-9327(99)01261-2
[18] V. A. Beckley et al., Pyrethrum Flowers: Kenya, a Better Source, Ind. Eng. Chem. 1938, 30, 835–838. https://doi.org/10.1021/ie50343a024
[19] T. F. West, The History of the African Pyrethrum Industry, J. Royal Soc. Arts 1959, 107, 423–441. https://www.jstor.org/stable/41366473
[20] Deutsche Parfümerie‐Zeitung 1920, 6, 117.
[21] A. Weed, Soap N.Y. 1938, 6, 133.
[22] C. Eagleson, Oil Synergist for Insecticides, US Patent 2,202,145, 1940.
[23] H. Wachs, Synergistic Insecticides, Science 1947, 105, 530–531. https://doi.org/10.1126/science.105.2733.530-a
[24] H. Wachs, O. F. Hedenburg, Methylenedioxyphenyl Cyclohexenones, J. Am. Chem. Soc. 1948, 70, 2216–2217. https://doi.org/10.1021/ja01186a067
[25] H. Wachs, O. F. Hedenburg, Preparation and Cyclization of Certain Insecticidally Active α-Acetyl-δ-keto Esters, J. Am. Chem. Soc. 1948, 70, 2695–2697. https://doi.org/10.1021/ja01188a022
[26] H. L. Haller et al., Effect of Sesamin and Related Compounds on the Insecticidal Action of Pyrethrum on Houseflies, J. Econ. Entomol. 1942, 35, 247–248. https://doi.org/10.1093/jee/35.2.247
[27] H. L. Haller et al., The Synergistic Action of Sesamin with Pyrethrum Insecticides, J. Org. Chem. 1942, 7, 183–184. https://doi.org/10.1021/jo01196a011
[28] C. B. Bernard, B. J. R. Philogène, Insecticide synergists: Role, importance, and perspectives, J. Toxicol. Environ. Health 1993, 38, 199–223. https://doi.org/10.1080/15287399309531712
[29] R. Brethnach in Piperonyl Butoxide (Ed: D. Glynne Jones), Academic Press, San Diego, USA, 1998. ISBN: 9780122869754
[30] G. F. Fuhrmann, Toxikologie für Naturwissenschaftler (in German), B. G. Teubner, Wiesbaden, Germany, 2006. ISBN: 9783835100244
The article has been published in German as:
- Von Insekten, Chrysanthemen und Menschen,
Klaus Roth, Elisabeth Vaupel,
Chem. unserer Zeit 2017, 51, 162–184.
https://doi.org/10.1002/ciuz.201700786
and was translated by W. E. Russey.
Pyrethrum: History of a Bio-Insecticide – Part 1
Chrysanthemum flowers as an insecticide
Pyrethrum: History of a Bio-Insecticide – Part 2
The chemical structures of pyrethrins and insecticide use between the World Wars
Pyrethrum: History of a Bio-Insecticide – Part 3
Comparing the insecticidal activity of DDT and pyrethrins
Pyrethrum: History of a Bio-Insecticide – Part 4
The comeback of pyrethrin research
Pyrethrum: History of a Bio-Insecticide – Part 5
The fall of DDT and the rise of pyrethrin derivatives
Pyrethrum: History of a Bio-Insecticide – Part 6
How pyrethrins kill insects
See similar articles by Klaus Roth published in ChemVistryiews Magazine




