Dried, pulverized flower heads of two chrysanthemum species (also known as “pyrethrum”) contain insecticides with nearly perfect toxicity profiles: highly toxic with respect to insects, but non-toxic to mammals. In this part, we look at the fall of DDT and the rise of pyrethrin derivatives.
12. The Demise of DDT
Although one might plausibly argue that DDT has rescued more people than any other compound ever (see Part 3), enthusiasm for its use gradually faded. This loss of acceptance was caused by DDT’s side effects, which became ever more manifest. For one thing, as a broad-band insecticide, DDT killed not only insect pests, but also their natural enemies. Almost more crucial, DDT also exterminated useful insects in general.
Moreover, in late summer 1945, Geigy in Basel, Switzerland, received first complaints regarding the sudden diminishing effectiveness for its DDT spray “Gesarol”, specifically with respect to barn flies. Soon thereafter, in 1946, the first DDT-resistant strains of house flies (Musca domestica) were discovered in northern Sweden [61], and in 1949, a first paper on the subject appeared in the journal Nature [62]. More and more insect species showed such resistance. At the same time, due to its great stability, DDT residues were found to be widely detectable within the environment; because of its lipid solubility, this was true even for fatty tissue in higher mammals.
12.1 Silent Spring
The “pro and con” debate regarding the use of DDT continued to be controversial among the experts, and was passionately discussed, although it remained largely unnoticed at first within the general public. This all changed, however, with the publication of Silent Spring in 1962, a nonfiction volume by the American biologist Rachel Carson (1907–1964) (see Fig. 16) [63]. In it, she warned of the consequences of excessive and indiscriminate dependence on insecticides, sketching out a vision that could class as apocalyptic:
“It was a spring without voices. On the mornings that had once throbbed with the dawn chorus of robins, catbirds, doves, jays, wrens, and scores of other bird voices there was now no sound; only silence lay over the fields and woods and marsh …. No witchcraft, no enemy action had silenced the rebirth of new life in this stricken world. The people had done it themselves.”
 |
|
Figure 16. Rachel Carson (1907–64), biologist and author of Silent Spring. |
In Silent Spring, Carson did not demand a rigorous prohibition of DDT, but argued “only” against its thoughtless and exorbitant usage:
“It is not my contention that chemical insecticides must never be used. I do contend that we have allowed poisonous and biologically potent chemicals to fall indiscriminately into the hands of persons largely or wholly ignorant of their potentials for harm.”
“No responsible person contends that insect-borne disease should be ignored. The question that has now urgently presented itself is whether it is either wise or responsible to attack the problem by methods that are rapidly making it worse. The world has heard much of the triumphant war against disease through the control of insect vectors of infection, but it has heard little of the other side of the story—the defeats, the short-lived triumphs that now support the alarming view that the insect enemy has been made actually stronger by our efforts. Even worse, we may have destroyed our very means of fighting.”
The effect of Silent Spring was especially immense within the American public, and it led to a long-term rethinking with respect to the evaluation of substances with environmental relevance. Within a few short years, the originally much-heralded “wonder-remedy” DDT became the veritable symbol for mankind’s destruction of nature. In 1972, production and use of this agent were finally forbidden in most of the world’s industrial nations.
12.2 Alternatives to DDT
Competing insecticides, of course, profited from the increasingly negative appraisal of DDT. This was especially true for the pyrethroids, which Rachel Carson had explicitly recommended in Silent Spring as a better alternative to DDT:
“The ultimate answer is to use less-toxic chemicals so that the public hazard from their misuse is greatly reduced. Such chemicals already exist: the pyrethrins, rotenone, ryania, and others derived from plant substances. Synthetic substitutes for the pyrethrins have recently been developed, and some of the producing countries stand ready to increase the output of the natural product as the market may require.”
Rotenone is an insecticidal constituent of barbasco (Deguelia utilis) or tuba (Derris elliptica) roots, ryania is a powder with insecticidal properties derived from the wood of the Ryania speciose willow, which constituted the active ingredient in the US insect control agents “Ryanex” and “Ryanicide”.
Rachel Carson’s express advice obviously carried weight: Carson’s remarks seem to have stimulated research into the pyrethroids generally [64], and modification studies soon began at every corner of the pyrethrin molecule.
13. Variations in the Pyrethrin Molecule
13.1 Variation within the Acidic Components
Further pyrethroid development [61] was not limited to the alcohol components alone (see Chapter 11.2). The chrysanthemic acid fragment was also modified. Here are the molecular sources of limited stability with respect to oxygen, heat, and light. The weak points themselves were identified as the two methyl groups on the double bond of the chrysanthemic acid fragment, which were subject to radical attack, whereby the methyl group at the trans-(E) position was the more sensitive of the two [65].
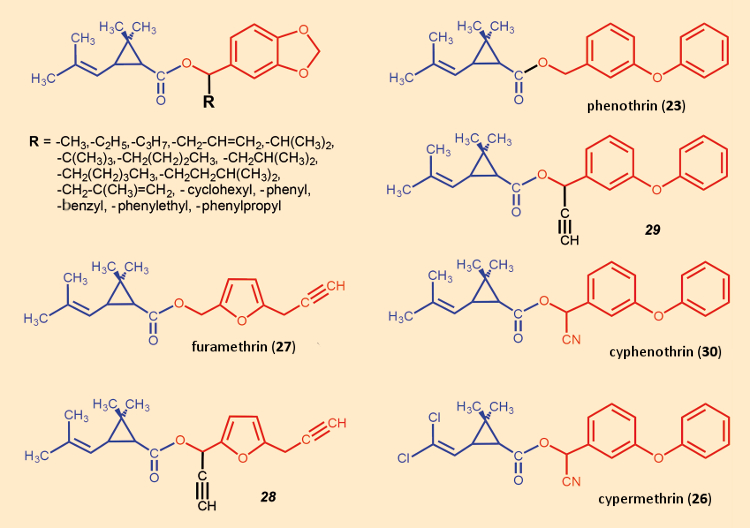
Hundreds of compounds with different substituents on the double bond were synthesized, with most being found to provide no improvement. Finally, it turned out that replacing the two relevant methyl groups by halogens was the remedy of choice. “Permethrin” (24), the dichloro derivative of phenothrin (23) (see Fig. 17), was the first pyrethroid with sufficient stability for agricultural use.
.jpg) |
|
Figure 17. Evolution of the pyrethroids. |
13.2 Variation at the α‐Position in the Alcohol Subunit
In the search for new structural variants, researchers were inspired by nature and, once again, by Staudinger und Ružička (see Chapter 6). In the natural product pyrethrin I (1), the alcohol component 4 is a secondary alcohol. Staudinger und Ružička’s weakly (but nevertheless somewhat) effective chrysanthemic acid piperonyl ester 19 (see Fig. 15) was based, on the other hand, on a primary alcohol. So, it seemed reasonable to add an additional substituent at the α‐position of piperonyl alcohol to transform it into a secondary alcohol. The idea was a reasonable one, but it completely missed the mark, since not in a single case (see Fig. 18) did it result in improved insecticidal properties [66].
 |
|
Figure 18. Pyrethroids with substituents in the α‐position. |
But a combination of luck and close observation brought the α‐position back into focus. In the course of developing an industrial procedure for preparing furamethrin (27), scientists at Sumitomo synthesized several byproducts that could perhaps have arisen as impurities during the manufacturing process. One of these was the diethynyl compound 28. Remarkably, this material showed greater insecticidal effectiveness than furamethrin itself. Apparently, an ethynyl group in the α‐position had increased the material’s potency.
Essentially the same synthetic modification was then applied to phenothrin (23), with the result that again the monoethynyl derivative 29 proved to be a more potent insecticide than the starting material. From this observation, exchanging the ethynyl group for the much more stable nitrile group was only a small, logical step. Operating on the same principle, and starting with the photostable permethrin (24), cypermethrin (26) was prepared, and this again displayed significantly increased insecticidal effectiveness.
A nitrile substituent in the α‐position transformed the originally primary alcohol into a secondary alcohol. This in itself, for steric reasons, would impede hydrolysis of the ester group. Moreover, the nitrile group is strongly electron-withdrawing. Both factors lead to the conclusion that pyrethroids with a nitrile function in the α‐position should, in vivo, be hydrolytically degraded only very slowly [67]. Due to a correspondingly increased half-life within an insect’s body, the compound’s toxicity would rise, and its LD50-dose fall.
With “Deltamethrin” (25) and “Cypermethrin” (26), two products appeared on the market in the mid-1970s that continue to this day to be economic successesful (see Figs. 17 and 18). Despite this triumph, it is worth recalling, however, that entry into the new class of pyrethroids was attributable originally to fear that a particular impurity might inadvertently be introduced in the course of furamethrin (27) manufacture.
References
[61] L. Straumann, Nützliche Schädlinge angewandte Entomologie, chemische Industrie und Landwirtschaftspolitik in der Schweiz 1874–1952 (in German), Chronos Verlag, Zürich, Switzerland, 2005. https://doi.org/10.3929/ethz-a-004757997
[62] J. Keiding, H. Van Deurs, D.D.T. resistance in houseflies in Denmark, Nature 1949, 163, 964–965. https://doi.org/10.1038/163964a0
[63] R. Carson, Silent Spring, Fawcett Crest, New York, USA, 1964. ISBN: 9780449014554
[64] K. Ramachandran, General Introduction to Synthetic Pyrethroids in Synthesis of pyrethroids based on 3 carene, Doctoral Thesis, Maharaja Sayajirao University of Baroda, India, 1985. http://shodhganga.inflibnet.ac.in (accessed July 2018)
[65] Y.-L. Chen, J. E. Casida, Photodecomposition of pyrethrin I, allethrin, phthalthrin, and dimethrin. Modifications in the acid moiety, J. Agric. Food Chem. 1969, 17, 208–215. https://doi.org/10.1021/jf60162a036
[66] W. F. Barthel, Synthetic pyrethroids, Adv. Pest Control Res. 1961, 4, 33.
[67] K. Mikata et al., Biotransformation and Enzymatic Reactions of Synthetic Pyrethroids in Mammals, Top. Curr. Chem. 2012, 314, 113–135. https://doi.org/10.1007/128_2011_254
The article has been published in German as:
- Von Insekten, Chrysanthemen und Menschen,
Klaus Roth, Elisabeth Vaupel,
Chem. unserer Zeit 2017, 51, 162–184.
https://doi.org/10.1002/ciuz.201700786
and was translated by W. E. Russey.
Pyrethrum: History of a Bio-Insecticide – Part 1
Chrysanthemum flowers as an insecticide
Pyrethrum: History of a Bio-Insecticide – Part 2
The chemical structures of pyrethrins and insecticide use between the World Wars
Pyrethrum: History of a Bio-Insecticide – Part 3
Comparing the insecticidal activity of DDT and pyrethrins
Pyrethrum: History of a Bio-Insecticide – Part 4
The comeback of pyrethrin research
Pyrethrum: History of a Bio-Insecticide – Part 5
The fall of DDT and the rise of pyrethrin derivatives
Pyrethrum: History of a Bio-Insecticide – Part 6
How pyrethrins kill insects
See similar articles by Klaus Roth published in ChemistryViews Magazine




