Dried, pulverized flower heads of two chrysanthemum species (also known as “pyrethrum”) contain insecticides with nearly perfect toxicity profiles: highly toxic with respect to insects, but non-toxic to mammals. In this part, we look at the comeback of pyrethrin research and the first synthetic derivatives of the pyrethrins.
11. Renaissance of Pyrethrum Research
11.1. Painstaking Structure Determination
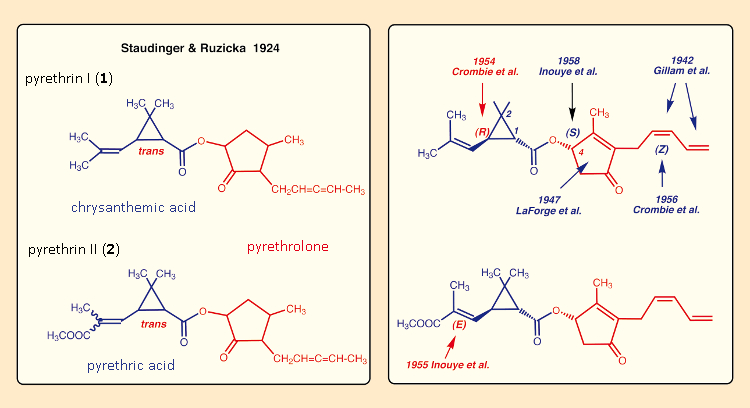
Despite the impressive successes of DDT, research institutes established in Japan, Great Britain, and the USA prior to the Second World War resumed their earlier research activities in the field of pyrethrins. With improved separation methods and new analytical approaches, it now proved possible to determine correct structures for the active ingredients in pyrethrum: in 1942, the locations of the two double bonds in the pyrethrolone sidechain were definitively established [37,38], and in 1947, the correct structure of the pyrethrolone five-membered ring was once and for all defined [39,40] (see Fig. 10). That meant—more than 30 years after Staudinger and Ružička’s efforts—the ability to tackle their original goal once again, from a scientifically sound basis: the synthesis of new pyrethrum derivatives.
The trans-relationship in the three-membered ring had already been established by Staudinger and Ružička, but it took until the mid-1950s before the configurational determination was completed for all the stereogenic carbon atoms as well as the double bonds in pyrethrin II [41]. The new assignments were later confirmed by X-ray analysis [42–46].
 |
|
Figure 10. The painstaking structure elucidations for pyrethrins I and II. |
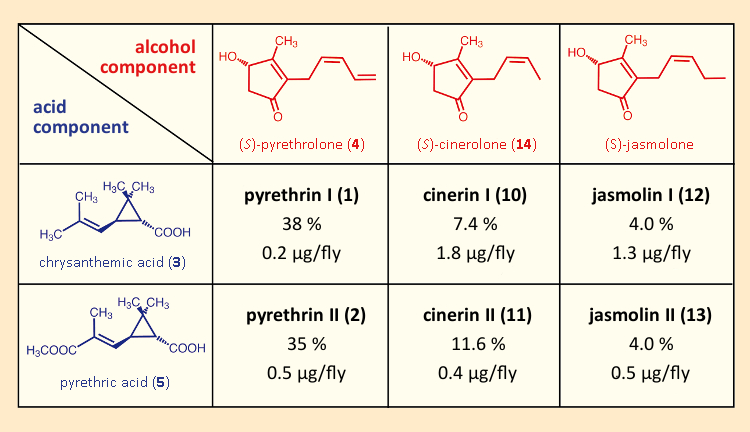
Thanks to improvements in separation methods, it also became possible to show that pyrethrum was a mixture, not of two, but rather of six structurally similar compounds. Frederick B. LaForge (1882–1958) and coworkers at the U.S. Bureau of Entomology and Plant Quarantine discovered the compounds cinerine I (10) and II (11) in 1944 and 1945 [47,48], and lastly, in 1966, P. J. Godin and coworkers identified jasmoline I (12) and II (13) [49]. All of these had insecticidal properties, differing only in the nature of the pyrethrolone sidechain (see Tab. 3). Also, it turned out that chrysanthemums had been withholding a special surprise: a synergistic effect that helped the natural pyrethrin mixture to offer a considerably higher toxicity than the sum of its individual components would have predicted [50,51].
|
Table 3. All the insecticidal substances in pyrethrum. The reported content levels of the six active insecticidal ingredients in pyrethrum correspond to a typical extract [52]. Reported LD50 values were determined using houseflies (Musca domestica). |
 |
11.2. Allethrin—the First Synthetic Derivative of the Pyrethrins
Unsuccessful Cinerin Synthesis
Although the number of known active insecticidal agents in pyrethrum had now grown to six, causing the overall picture to become more confusing, the newly discovered cinerins actually proved to be a godsend. All the previous synthetic efforts had focused exclusively on pyrethrin I (1). Now it turned out, however, that thanks to the absence of a second double bond in the pyrethrolone side-chain, cinerin I (10) presented a much simpler synthetic goal.
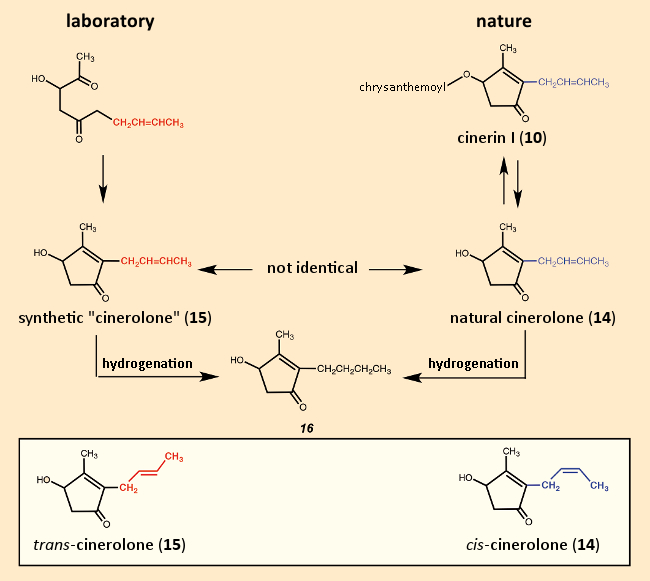
For their total synthesis of cinerin I (10), LaForge and his coworkers proposed first preparing the five-membered ring segment (see Fig. 11). It must have come as a great disappointment when their synthetic “cinerolone” (15) turned out not to be identical to “natural” cinerolone (14). Since both “cinerolones” gave the same hydrogenation product, they must have been stereoisomers, differing in the configuration of the double bond in the side chain. The synthetic product here had the trans– configuration, whereas the natural product is cis-configured [53,54]. It could, of course, have been the reverse, but the decisive double bond was introduced by way of trans-crotyl bromide (CH3‐CH=CH‐CH2Br). Thus, the first attempt at a cinerin I total synthesis failed due to a cis/trans isomer issue. The first true total synthesis of cinerin I was not accomplished until 1950 [55].
 |
|
Figure 11. Unsuccessful attempt at a first cinerin total synthesis in 1949. |
Allethrin
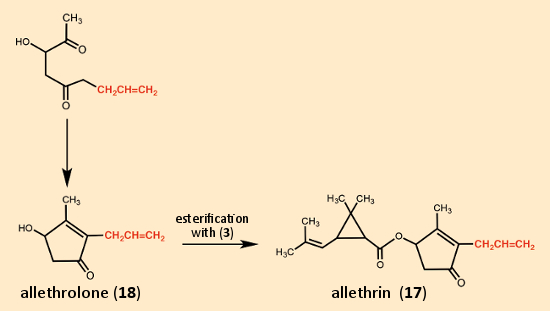
LaForge and his coworkers then had an ingenious idea. They avoided the difficulties with cis/trans isomerism in the side-chain by simply eliminating the methyl group at the end, so that there was no longer any possibility for isomerism. The synthesis they developed for cinerin I (10) was sufficiently flexible to allow preparation of this new compound as well (see Fig. 12). By introducing an allyl residue (marked in red in Figure 12) instead of a crotonyl residue (red in Figure 11), the five-membered ring constituent referred to as allethrolone (18) was prepared, which, when esterified with chrysanthemic acid (3), gave allethrin (17).
 |
|
Figure 12. Allethrin, the first synthetic pyrethroid. |
The first synthetic pyrethroid, the new material known as allethrin (17), proved just as insecticidal as pyrethrin (1) or cinerin (10). The U.S. Department of Agriculture proudly announced the scientific breakthrough on March 11, 1949. Excitement regarding this success was so great because, shortly before it occurred, there was also report of a successful synthesis of chrysanthemic acid (3) [56,57]. Both of the essential molecular components were now readily accessible. The Japanese firm Sumitomo Chemical Ltd. developed an industrial-scale synthesis based on the work of LaForge, and began marketing allethrin (17) as the first of the commercial “pyrethroids” in 1954.
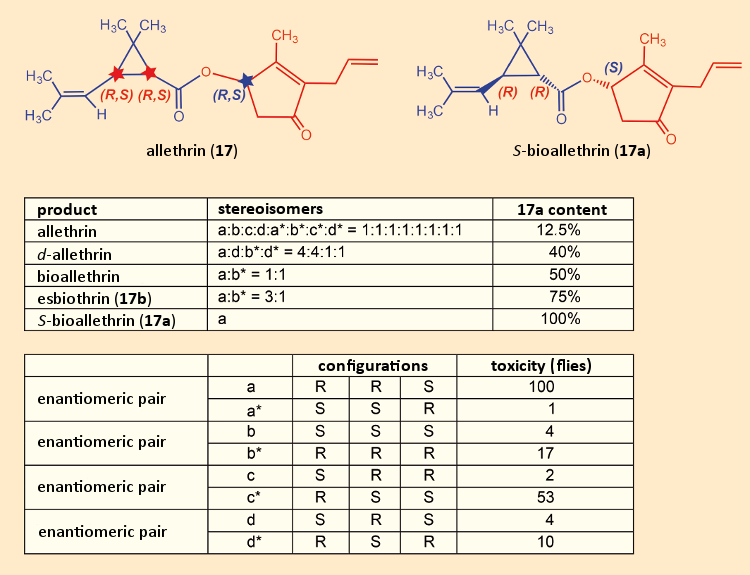
Allethrin (17) was plagued by certain fundamental problems associated with this class of substances. It contained three stereogenic carbon atoms, two in the three-membered ring, and one in the five-membered ring. That meant there was a possibility of eight stereoisomers (see Fig. 13). Since the industrial synthesis began with a cis/trans-mixture of chrysanthemic acids (3), and was not stereoselective, the potential mixture of eight stereoisomers was, in fact, obtained. At laboratory scale, it could be separated, and the insecticidal activity of each of the various allethrin isomers could be determined with respect to houseflies (Musca domestica) [58].
Isomer 17a was shown to be the most potent. The industrial preparation was then optimized stepwise in an attempt to increase the content of this particular isomer. It first became possible to achieve a 17a content in esbiothrin (17b) of 75 %. Eventually, it proved possible to develop a synthesis of pure 17a, which shortly thereafter was marketed under the tradename S-Bioallethrin.
 |
|
Figure 13. Insecticidal effectiveness of each of the allethrin stereoisomers. |
Varying the Alcohol Components
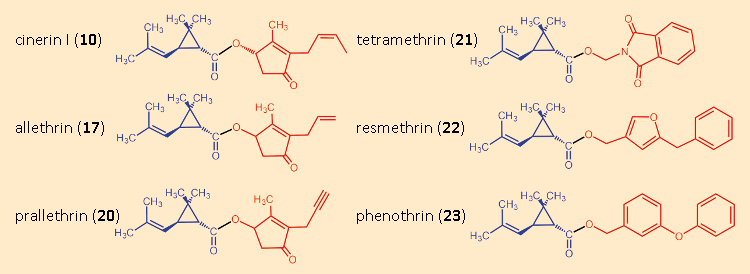
Allethrin (17) proved that there could indeed be other highly effective synthetic derivatives of the pyrethrins. One had simply to find them. Warned by Staudinger and Ružička’s disappointments, but also bearing LaForge’s allethrin success in mind, researchers initially ventured to undertake only very minor structural modifications (see Fig. 14).
 |
|
Figure 14. Variations in the alcohol components. |
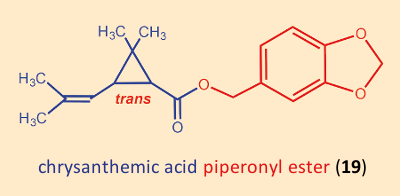
Hidden among the many ineffective products that Staudinger und Ružička had prepared was a substituted benzyl ester, the piperonyl ester of chrysanthemic acid (19) (see Fig. 15). It actually displayed only minimal insecticidal activity, but was not completely without effect. Thus, one began the esterification of chrysanthemic acid (3) with all sorts of benzyl alcohols. The first pyrethroids were derived from natural cinerin I (10), and were chrysanthemic acid (3) esterified with various alcohols. Tetramethrin (21), introduced in 1965, was the first such insecticide without a stereogenic center in the alcohol component.
 |
|
Figure 15. Piperonyl ester of chrysanthemic acid. |
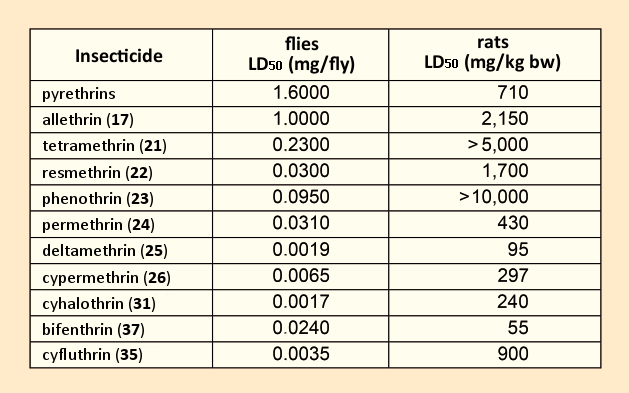
Tetramethrin (21) marked the first use of an alcohol component that lacked any stereocenter, a significant advance toward economical synthesis, since this meant avoiding the stereocenter of the carbon atom in α position to the OH group. Relative to the natural product, several representatives of this first pyethroid generation displayed better toxicological characteristics (see Tab. 4) [59,60]. However, with respect to agricultural applications, the desired breakthrough had still not been achieved, because these first pyrethroids were just as unstable as the natural products.
This comparison applies only to toxicological properties, however. Mere independence with respect to a seasonally harvested raw material, one necessarily imported from far away, should not be underestimated from an economic standpoint. A domestic synthetic product meant reliable availability regardless of season, an important prerequisite for product innovation in a processing industry.
|
Table 4. Toxicities of various pyrethroids [59,60]. |
 |
References
[37] A. E. Gillam, T. F. West, Absorption spectra and the structures of pyrethrins I and II, J. Chem. Soc. 1942, 671–676. https://doi.org/10.1039/jr9420000671
[38] A. E. Gillam, T. F. West, Absorption spectra and the structure of pyrethrins I and II. Part II, J. Chem. Soc. 1944, 49–51. https://doi.org/10.1039/jr9440000049
[39] F. B. LaForge, S. B. Soloway, The Structure of Dihydrocinerolone, J. Am. Chem. Soc. 1947, 69, 186. https://doi.org/10.1021/ja01193a505
[40] F. B. LaForge, S. B. Soloway, Constituents of Pyrethrum Flowers. XXI. Revision of the Structure of Dihydrocinerolone, J. Am. Chem. Soc. 1947, 69, 2932–2935. https://doi.org/10.1021/ja01204a003
[41] Y. Inouye et al., Studies on Synthetic Pyrethroids. Part V. Synthesis of Geometrical Isomers of Chrysanthemum Dicarhoxylic Acid, Bull. Agr. Chem. Soc. Jpn. 1955, 19, 193–199. https://doi.org/10.1080/03758397.1955.10857288
[42] L. Crombie et al., The Chrysanthemumcarboxylic Acids. Part VI. The Configurations of the Chrysanthemic Acids, J. Chem. Soc. 1954, 470–475. https://doi.org/10.1039/jr9540000470
[43] L. Crombie et al., Experiments on the synthesis of the pyrethrins. Part X. Intermediates for the synthesis of cis-pyrethrolone, J. Chem. Soc. 1956, 126–135. https://doi.org/10.1039/jr9560000126
[44] Y. Katsuda et al., The Absolute Configuration of Naturally Derived Pyrethrolone and Cinerolone, Bull. Agr. Chem. Soc. Jpn. 1958, 22, 427–428. https://doi.org/10.1080/03758397.1958.10857510
[45] M. J. Begley et al., Absolute configuration of the pyrethrins. Configuration and structure of (+)-allethronyl (+)-trans-chrysanthemate 6-bromo-2,4-dinitrophenylhydrazone by X-ray methods, J. Chem. Soc. D 1972, 23, 1276–1277. https://doi.org/10.1039/c39720001276
[46] L. Crombie et al., Structural and physical aspects of pyrethrum chemistry, Pestic. Sci. 1976, 7, 225–230. https://doi.org/10.1002/ps.2780070303
[47] F. B. LaForge, W. F. Barthel, Constituents of Pyrethrum Flowers. XVI. Heterogeneous Nature of Pyrethrolone, J. Org. Chem. 1944, 9, 242–249. https://doi.org/10.1021/jo01185a006
[48] F. B. LaForge, W. F. Barthel, Constituents of Pyrethrum Flowers. XIX. The Structure of Cinerolone, J. Org. Chem. 1945, 10, 222–227. https://doi.org/10.1021/jo01179a011
[49] P. J. Godin et al., The jasmolins, new insecticidally active constituents of Chrysanthemum cinerariaefolium VIS, J. Chem. Soc. C 1966, 332–34. https://doi.org/10.1039/J39660000332
[50] D. C. Sheppard, B. Swedlund, Toxicity of Individual Pyrethrin Esters to House Flies (Diptera: Muscidae), J. Entomol. Sci. 2000, 35, 279–282. https://doi.org/10.18474/0749-8004-35.3.279
[51] A. S. Gunasekara, Environmental Fate of Pyrethrins, www.cdpr.ca.gov, 2004. (accessed July 2018)
[52] A. Glynne‐Jones, Pyrethrum, Pestic. Outlook 2001, 12, 195 198. https://doi.org/10.1039/b108601b
[53] M. S. Schechter et al., The Synthesis of Cyclopentenolones of the Type of Cinerolone, J. Am. Chem. Soc. 1949, 71, 1517–1517. https://doi.org/10.1021/ja01172a531
[54] M. S. Schechter et al., Constituents of Pyrethrum Flowers. XXIII. Cinerolone and the Synthesis of Related Cyclopentenolones, J. Am. Chem. Soc. 1949, 71, 3165–3173. https://doi.org/10.1021/ja01177a065
[55] L. Crombie, S. H. Harper, Experiments on the synthesis of the pyrethrins. Part IV. Synthesis of cinerone, cinerolone, and cinerin-I, J. Chem. Soc. 1950, 1152–1160. https://doi.org/10.1039/jr9500001152
[56] I. G. M. Campbell, S. H. Harper, Experiments on the synthesis of the pyrethrins. Part I. Synthesis of chrysanthemum monocarboxylic acid, J. Chem. Soc. 1945, 283–286. https://doi.org/10.1039/jr9450000283
[57] K. Ujihara et al., Recent Advances of Pyrethroids for Household Use, Top. Curr. Chem. 2012, 314, 31–48. https://doi.org/10.1007/128_2011_253
[58] L. Crombie, M. Elliott, Chemistry of the Natural Pyrethrins, Progr. Chem. Org. Nat. Prod. 1961, 19, 120–164.
[59] M. Elliott in Crop Protection Agents from Nature (Ed: L. G. Copping), The Royal Society of Chemistry, London, 1996. ISBN: 0854044140
[60] R. Tsuji et al., Mammal Toxicology of Synthetic Pyrethroids, Top. Curr. Chem. 2012, 314, 83 –111. https://doi.org/10.1007/128_2011_269
The article has been published in German as:
- Von Insekten, Chrysanthemen und Menschen,
Klaus Roth, Elisabeth Vaupel,
Chem. unserer Zeit 2017, 51, 162–184.
https://doi.org/10.1002/ciuz.201700786
and was translated by W. E. Russey.
Pyrethrum: History of a Bio-Insecticide – Part 1
Chrysanthemum flowers as an insecticide
Pyrethrum: History of a Bio-Insecticide – Part 2
The chemical structures of pyrethrins and insecticide use between the World Wars
Pyrethrum: History of a Bio-Insecticide – Part 3
Comparing the insecticidal activity of DDT and pyrethrins
Pyrethrum: History of a Bio-Insecticide – Part 4
The comeback of pyrethrin research
Pyrethrum: History of a Bio-Insecticide – Part 5
The fall of DDT and the rise of pyrethrin derivatives
Pyrethrum: History of a Bio-Insecticide – Part 6
How pyrethrins kill insects
See similar articles by Klaus Roth published in ChemistryViews Magazine




