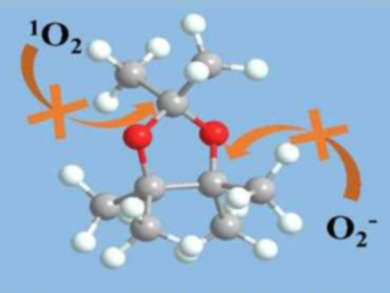
Ether-based electrolytes are widely used in lithium-oxygen batteries because of their relatively high ionic conductivity, O2 solubility, and electrochemical stability. However, conventional ethers have weak points, particularly the α-H atoms next to the ether bonds. Intermediates formed during the discharge/charge processes, such as O2– or 1O2, can cause hydrogen abstraction and, thus, decomposition of the electrolyte.
Yue Shen, Yunhui Huang, Huazhong University of Science and Technology, Wuhan, China, and colleagues have developed a fully methylated cyclic ether, 2,2,4,4,5,5-hexamethyl-1,3-dioxolane (HMD, pictured), in which all α-H atoms are replaced with methyl groups and unwanted hydrogen abstraction is avoided. HMD was synthesized via a one-step reaction between pinacol and 2,2-dimethoxypropane.
HMD shows excellent stability in the presence of O2– or 1O2. Side reactions at the cathode side are significantly suppressed. The cycle life of a cell with an HMD-based electrolyte is four times longer than for a cell with conventional ethers. According to the researchers, the findings could lead to the development of high-performance rechargeable lithium-oxygen batteries.
- A stable lithium-oxygen battery electrolyte based on fully methylated cyclic ether,
Zhimei Huang, Haipeng Zeng, Meilan Xie, Xing Lin, Zhaoming Huang, Yue Shen, Yunhui Huang,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201812983