Water electrolysis driven by renewable energy can produce “clean” hydrogen fuel. The deployment of this technology on a large scale still requires cost-reduction. Aqueous solutions at near-neutral pH, for example, can be more cost-effective than the existing electrolyzers that are operated in extreme-pH mediums However, systems at near-neutral pH suffer from lower efficiency. Adding buffer species and optimizing the physicochemical properties of the solutions could improve this efficiency.
Kazuhiro Takanabe, The University of Tokyo, Japan, and colleagues have shown that electrolysis performance in highly concentrated aqueous buffer solutions can be comparable to the extreme-pH counterparts. The team assessed the properties of concentrated buffer electrolytes (Li, Na, K, and Cs phosphate) at various temperatures and molalities, i.e., solubility, viscosity, and conductivity. They also determined the efficiency losses associated with mass-transport during water electrolysis.
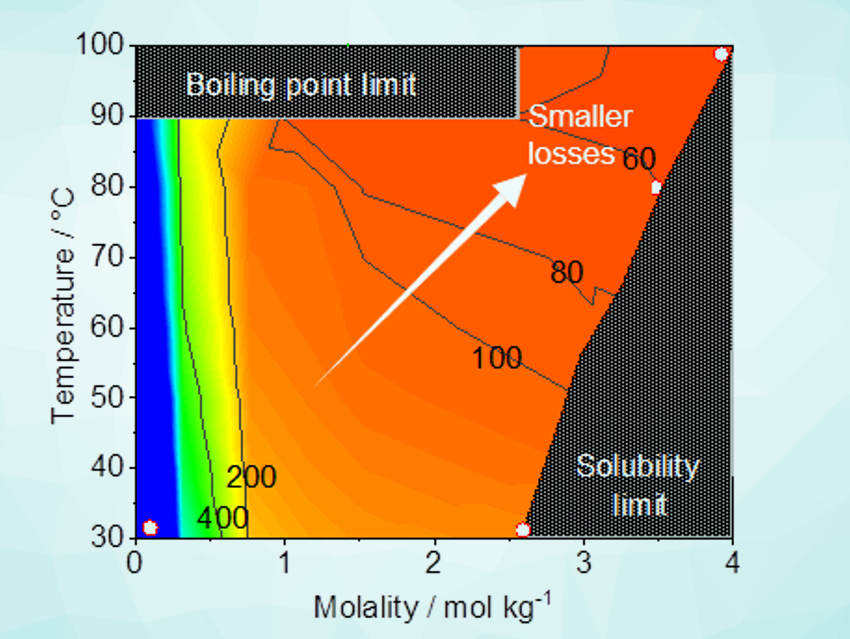
The researchers found that saturated potassium phosphate solutions at 80 °C–100 °C are optimal to minimize the losses originating from mass-transport at neutral pH. The water electrolysis performance over model electrodes (IrOx and Pt as anode and cathode, respectively) was comparable to the performance of systems at extreme pH values. The concentrated buffer solution also showed improved electrochemical stability. The work shows that highly concentrated buffer solutions should be regarded as an alternative environment for water electrolysis.
- Water Electrolysis in Saturated Phosphate Buffer at Neutral pH,
Takahiro Naito, Tatsuya Shinagawa, Takeshi Nishimoto, Kazuhiro Takanabe,
ChemSusChem 2020.
https://doi.org/10.1002/cssc.202001886