After discovering the urea cycle, Hans Adolf Krebs, a 32-year-old scientist was forced to leave his homeland. Just one year earlier, freshly habilitated Adjunct Professor Krebs (see Fig. 1) had figured out how mammals convert ammonia, a toxic product of protein decomposition, into harmless urea. This scientific feat earned him international recognition and high praise from the Dean of the medical school in Freiburg, Germany. Just four months later, the same Dean furloughed Krebs with immediate effect, in compliance with Ministerial Regulation A 7642. What exactly happened in 1933?
 |
|
Figure 1. Hans Adolf Krebs. |
1. Childhood and Life up to 1932
We will begin by retracing a short interval in the life of young Hans Krebs. Personally, he viewed himself as “lucky” [1]. He grew up in a sheltered home in Hildesheim, Germany. His father was an ear, nose, and throat physician, and his mother raised Hans and his two siblings. Both parents came from Jewish families that had dwelled in Germany for centuries, though religion played no role in the upbringing of the children.
Krebs’ father was convinced that there was only one answer for the latent antisemitism that had been present in all countries for centuries: All Jewish people had to fully assimilate and give up their beliefs. He thus gave his children typically German names (Elisabeth, Hans, and Wolfgang), allowed them no Jewish religious education, and sent them to evangelical religious classes, which was required in Germany at the time.
 From an early age, Hans Krebs was certain that he wanted to become a doctor. He studied medicine in Göttingen, Freiburg, and Munich, all Germany, earning his doctorate with the anatomist von Möllendorf in Freiburg. His interest in scientific research was awakened, but Krebs was not yet sure that he could be successful on this path. He worked as an assistant to Otto Warburg at the Kaiser Wilhelm Institute for Biology in Berlin-Dahlem, Germany. At the end of this job, Warburg advised him to stay away from a career in biochemistry and to become a practicing physician: “Biochemistry is not a profession, but medicine is. If you decide to marry and wish to buy a house, you will probably go to a bank to ask for a loan. The bank will ask if you have a profession and if you say you are a biochemist, you won’t get a penny; but if you say you are a doctor, you will probably get everything you want [1].”
From an early age, Hans Krebs was certain that he wanted to become a doctor. He studied medicine in Göttingen, Freiburg, and Munich, all Germany, earning his doctorate with the anatomist von Möllendorf in Freiburg. His interest in scientific research was awakened, but Krebs was not yet sure that he could be successful on this path. He worked as an assistant to Otto Warburg at the Kaiser Wilhelm Institute for Biology in Berlin-Dahlem, Germany. At the end of this job, Warburg advised him to stay away from a career in biochemistry and to become a practicing physician: “Biochemistry is not a profession, but medicine is. If you decide to marry and wish to buy a house, you will probably go to a bank to ask for a loan. The bank will ask if you have a profession and if you say you are a biochemist, you won’t get a penny; but if you say you are a doctor, you will probably get everything you want [1].”
Krebs took this advice and went to Hamburg-Altona, Germany, to work as a resident doctor. However, he quickly discovered that he missed the stimulating atmosphere of a scientific environment. He left Hamburg and took a position as a doctor with Siegfried Thannhauser at the University of Freiburg Hospital, Germany. There, he was responsible for all of the patients in an entire ward, but Thannhauser also provided him with a laboratory, in which he could pursue independent scientific research for the first time.
Krebs’ first medical graduate student, Kurt Henseleit, proved to be an extremely talented experimentalist. They worked together very hard and harmoniously, which led to the sensational discovery of the urea cycle in 1932. This allowed Krebs to qualify as a Professor and obtain his teaching permit on December 28, 1932.
2. Why the Urea Cycle Made Hans Krebs Famous
The fats and carbohydrates we consume daily are broken down in the body to form carbon dioxide and water or, in the case of a surplus, stored as body fat or glycogen. In contrast, excess amino acids that are ingested as proteins cannot be stored and must be fully degraded. This causes large amounts of toxic ammonia, NH3, to accumulate, which the body must convert to less toxic urea and expel.
Humans produce about 30 g of urea (1) every day, a tremendous amount that can rise threefold (1.5 mol) for a protein-rich diet. Humans thus synthesize more urea (in mol) per day than glucose (100 g/d = 0.55 mol) [2].
2.1. How Does the Body Synthesize Such a Huge Amount of Urea Daily?
From a chemical point of view, the whole thing can be summarized with a simple overall reaction:
|
|
(1) |
However, how this synthesis occurred within cells was one of the biggest mysteries of biochemistry in 1930.
Working with Otto Warburg at the Kaiser Wilhelm Institute of Biology in Berlin-Dahlem, Hans Krebs had thoroughly mastered the study of metabolic processes in fresh tissue sections. While Warburg was interested in the decomposition sequences for substances such as glucose, Krebs made a lucky decision when embarking on his first independent research in Freiburg. He chose to apply the methodology he had learned to a synthetic problem: the conversion of ammonia to urea.
2.2. Krebs’ Measurement of Urea Synthesis
We will first trace a typical measurement of urea synthesis in a tissue sample as Krebs would have carried it out in Freiburg in the early 1930s: A tissue sample of about 0.3 mm in thickness, or about 50 cell layers, was prepared from a fresh organ and vigorously shaken in a nutrient solution. The thin layer of the section ensured an adequate supply of nutrients and oxygen to all of the cells. To study the influence of a compound on the production of urea, that compound was added to the nutrient solution and the tissue section was shaken in it for an hour.
After the tissue section was removed from the nutrient solution, the enzyme urease was added. This enzyme splits any urea present into ammonia and carbon dioxide, in a reversal of reaction (1).
|
|
(1) |
While the ammonia remains dissolved in water, the carbon dioxide causes the pressure to increase within the closed apparatus. This can be measured using a manometer and the amount of urea originally formed can be calculated. This is a highly simplified description of the experimental procedure.
2.3. Development of this Method of Measurement
This simple-sounding method of measurement did not fall into Krebs’ lap: he had to develop it. First, he had to determine the optimal conditions (temperature, pH value, etc.) for the urease reaction and refine the experimental process in a series of tests so that the amount of urea could indeed be quantitatively determined from the observed increase in pressure. A second difficulty lay in the fact that metabolism in tissue samples could only be maintained for a limited amount of time with the conventional Ringer solution of the time. This led to highly scattered data and not very reliable measurements.
It is surely one of Krebs’ greatest accomplishments that he refined the “physiological” table salt preparation first developed by Ringer and modified by Warburg by bringing it as close to natural mammalian plasma as possible (see Tab. 1). This medium, later named Krebs-Ringer solution, proved to be superior in every aspect. With it, Krebs laid the foundation for reproducible measurements in studies of tissue sections. This important methodological innovation made the corresponding publication from 1932 [3] one of the most cited works of biochemistry, even decades later [4].
|
Table 1. “Physiological” table salt solutions. (The chloride content seems high at first glance, but is necessary to balance the charge of the cations. In plasma, this is achieved by the anionic side-chains of proteins and lactate.) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(all ion concentrations in mg ion/100 cm3 solution) |
2.4. The Experiments
Methodologically armed in this way, Krebs was able to experimentally study the production of urea. When he started his work, the following facts were considered verified:
- It was known that in the first step of the degradation of excess amino acids, ammonia is split off (deamination). The ammonia is then excreted as urea.
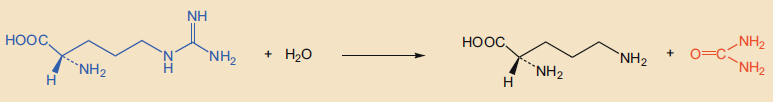
- Liver tissue contains large amounts of the highly active enzyme, arginase, which hydrolytically splits the amino acid arginine (2) into ornithine (3) and urea (1), according to reaction equation (2).
|
|
(2) |
This equation would seem to solve the problem. Let us assume that humans synthesize their urea exclusively from arginine. A daily output of 30 g of urea would require the hydrolyzation of about 90 g of arginine. Such an enormous quantity of arginine can neither be synthesized nor ingested in the form of food by a person. In other words: Reaction (2) can only be responsible for a very tiny portion of the urea production. Most of it has to come from somewhere else. This was the scientific consensus at the time.
At the beginning of his experiments, Krebs had no working hypothesis, no vision [5,6]. His lab notebooks, as well as those of his doctoral student Kurt Henseleit, document the impressive number and variety of compounds they tested. Like searching through a haystack, Krebs examined all possible amino acids and always observed significantly increased production of urea after their addition. This increase was particularly high for arginine, which is not surprising, given equation (2), by which it is known that arginine is directly broken down into urea.
On October 26, 1931, he tested a mixture of amino acids and additional ammonia for the first time and was surprised to discover that urea production was higher than with only one of the two components. Krebs changed his strategy and checked the production of urea from ammonia after addition of all kinds of different compounds, such as glucose, pyruvate, lactate, and fructose.
On November 15, 1931, Henseleit almost incidentally ended a long series of experiments by testing the amino acid ornithine (3). The result was surprising because addition of ammonia and ornithine raised urea production by a great deal. Neither ammonia nor ornithine alone were so active. This was the experimental breakthrough, though its implications were not immediately clear.
2.5 Ornithine Effect and Citrulline
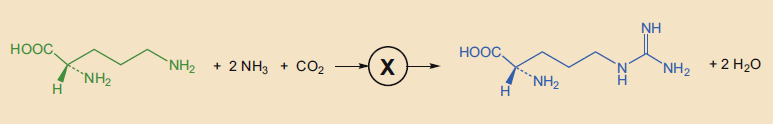
Starting on January 14, 1932, Krebs and Henseleit varied the concentration of ornithine and determined that they could dip far under the stoichiometrically required amount, to the point that only one molecule of ornithine was needed to form 20 molecules of urea. Furthermore, ornithine was not consumed at all during the overall conversion. This observation, designated the ornithine effect, can be described by reaction equation (3), in which ornithine acts as a catalyst for urea formation.
|
|
(3) |
In their first publication [7], Henseleit and Krebs summarized everything known about the catalytic effect of ornithine at the time in reaction equations (4) and (5). They knew that at least one further intermediate, “X”, must be passed through, because it is extremely unlikely that a total of four molecules would react with each other simultaneously, as shown in reaction (4).
|
|
(4) |
|
|
(5) |
With a little “paper chemistry”, Krebs was able to identify a possible candidate: citrulline (4). This compound was isolated from watermelons (Citrullis vulgaris) in 1914 and can be made from arginine by either bacterial decomposition [8] or chemical synthesis [9].
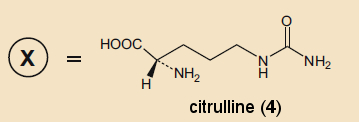
Hans Krebs requested small samples from the two only research groups that had citrulline on hand [10]. He obtained
samples from both and used them to immediately prove that arginine, ornithine, and citrulline all substantially catalytically accelerate the formation of urea from ammonia.
This made everything clear! A few days after carrying out these experiments, on June 12, 1932, Krebs sent his manuscript to Hoppe-Seyler’s Zeitschrift für Physiologie [3]. In this paper, he combined the results of the three reaction equations (see Fig. 2).
 |
|
Figure 2. The urea cycle in the original publication [3] and the cyclic form published later [11]. |
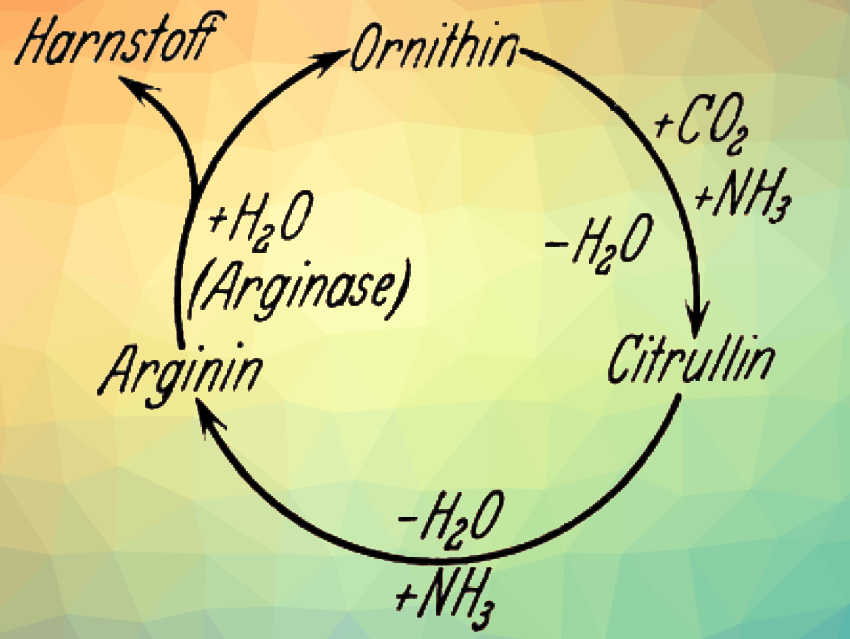
2.6. Depiction of the Urea Cycle
The familiar and impressive circular depiction of the urea cycle was introduced by Krebs as “ghostwriter” in a 1933 publication by this doctoral student, Hildegard Manderscheid [11]. When asked why he did not depict his results as a circle in his original publication, Krebs replied that everything was there in the publication [12]. For him, the circular arrangement of the compounds only served to make the overall reaction sequence easier to understand.
But this was just the beginning! The ways in which this first ornithine cycle was refined over the years is extensively depicted in every modern biochemistry textbook.
3. Scientific Breakthrough and Forced Leave
This scientific breakthrough pushed Hans Krebs into the top tier of up-and-coming German scientists. He received numerous invitations—from Otto Meyerhof at the Heidelberg Kaiser Wilhelm Institute for Medical Research and even from the Director of the Kaiser Wilhelm Society, Max Planck, to the “Dahlem Medical Soiree” in Berlin. A promising academic future lay before him.
On the way back from Berlin, he visited his father, stepmother, and ten-month-old half-sister Gisela in Hildesheim. He had no idea that he would never see his father again.
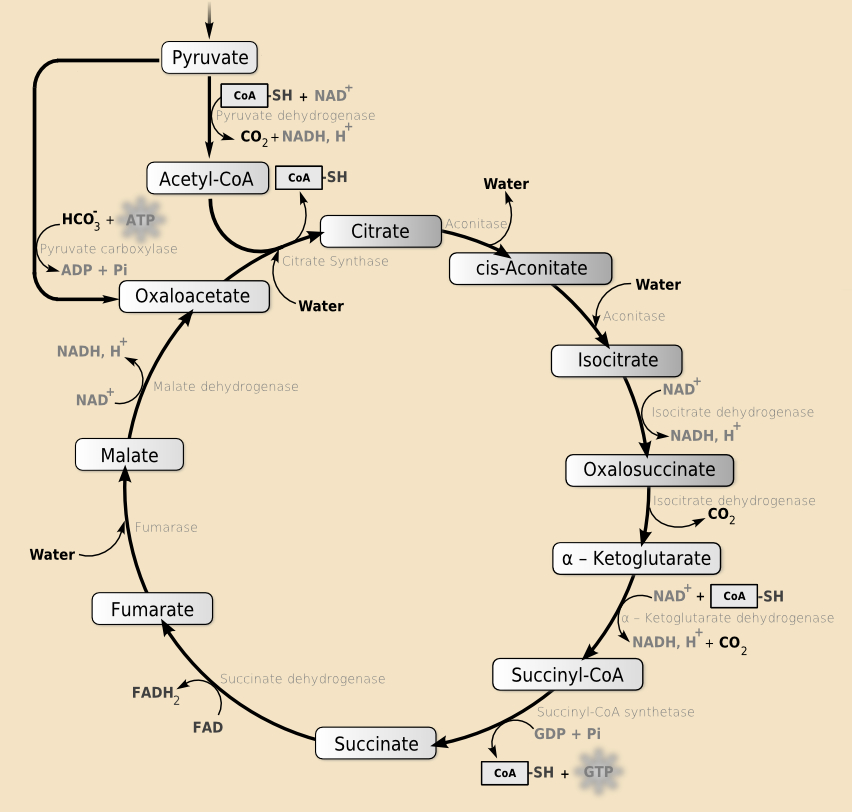
After completing his work on the urea cycle, Krebs turned his efforts to the degradation of amino acids. He also began to study the oxidative degradation of dicarboxylic acids. Years later, these efforts would lead to his most significant discovery: the citric acid cycle or Krebs cycle (see Fig. 3).
 |
|
Figure 3. Citric acid cycle or Krebs cycle (based on an image by YassineMrabet, wikimedia commons, CC BY-SA 3.0). |
However, dark brown clouds began to cover the skies over Germany; clouds that were not noticed by most people. Even Krebs saw no danger. Although he had heard about occasional antisemitic hostility and discrimination, he had never experienced them himself. How could he have, since his whole family was completely non-religious? Only after Hitler seized power did Hans Krebs grasp that he belonged to a persecuted group to which he had never felt he belonged. Indeed, it was Hitler who made Hans Krebs Jewish.
In Part 2, we will take a closer look at how the academic world around Hans Krebs changed, how his colleagues, his university, and finally all of Germany changed so much that he had to leave his homeland.
References
[1] H. A. Krebs, Reminiscences and Reflections, Oxford University Press, Oxford, UK, 1981. ISBN: 978-0-198-54702-0
[2] K. Roth, Die Stöchiometrie der Olympischen Spiele: citius, altius, fortius – schneller, höher, stärker, Chem. Unserer Zeit 2008, 42, 270–280. https://doi.org/10.1002/ciuz.200800467
[3] H. A. Krebs, K. Henseleit, Untersuchungen über die Harnstoffbildung im Tierkörper (in German), Hoppe-Seyler’s Z. Physiol. Chem. 1932, 210, 33–66. https://doi.org/10.1515/bchm2.1932.210.1-2.33
[4] This Week’s Citation Classic: Krebs H. A. & Henseleit K. Studies on urea formation in the animal organism, garfield.library.upenn.edu. (accessed October 31, 2020)
[5] G. Graßhoff, et al., Zur Theorie des Experiments (in German), Bern Studies History and Philosophy of Science, Bern, Switzerland, 1981. ISBN: 978-3-898-11827-9
[6] G. Graßhoff, K. Nickelsen, Dokumente zur Entdeckung des Harnstoffzyklus (in German), Bern, Switzerland, 1981. ISBN: 978-3-831-11416-0
[7] H. A. Krebs, K. Henseleit, Untersuchungen über die Harnstoffbildung im Tierkörper (in German), Klin. Wochenschr. 1932, 11, 757–759. https://doi.org/10.1007/BF01757657
[8] D. Ackermann, Über den biologischen Abbau des Arginins zu Citrullin (in German), Hoppe-Seyler’s Z. physiol. Chem. 1931, 203, 66–69. https://doi.org/10.1515/bchm2.1931.203.1-2.66
[9] M. Wada, Über Citrullin, eine neue Aminosäure im Preßsaft der Wassermelone, Citrullus vulgaris schrad. (in German), Biochem. Z. 1930, 224, 420.
[10] H. A. Krebs, The discovery of the ornithine cycle of urea synthesis, Trends Biochem. Sci., 1982, 76–78. https://doi.org/10.1016/0968-0004(82)90083-4
[11] H. Manderscheid, Über die Harnstoffbildung bei den Wirbeltieren (in German), Biochem. Z. 1933, 263, 245.
[12] F. L. Holmes, Hans Krebs, Oxford University Press, Oxford, UK, 1993. ISBN: 978-0-195-07072-9
[13] L. Jaenicke, Profile der Biochemie (in German), S. Hirzel Verlag, Stuttgart, Germany, 2007. ISBN: 978-3-7776-1517-2
The article has been published in German as:
- Dann machte ich mich allein auf den Weg, um den 11‐Uhr‐Zug zu erreichen. Sir Hans Adolf Krebs (1900–1981),
Klaus Roth,
Chem. unserer Zeit 2008, 42, 346–359.
https://doi.org/10.1002/ciuz.200800471
and was translated by Caroll Pohl-Ferry.
Sir Hans Adolf Krebs (1900 – 1981) – Part 2
The life of the discoverer of the citric acid and urea cycles from 1933 to 1981
Sir Hans Adolf Krebs (1900 – 1981) – Part 3
The Nobel Prize in Physiology or Medicine in 1953 and Krebs’ thoughts about life as a displaced person (will be published in January 2021)
See similar articles by Klaus Roth published on ChemistryViews.org
Also of Interest
- Video: Great Architecture and Chemists in Dahlem,
Vera Koester,
ChemistryViews.org 2018.
Walking tour through Dahlem, one of the richest parts of Berlin and an important historical center for academic research

.jpg)