Nutmeg is generally used freshly grated in the kitchen because its aroma is slightly volatile. Nutmeg oil is used in the food industry because of its easier dosage and longer shelf life. The spice is used, for example, in potato dishes, soups, and pastries. The yellow-orange pulp is used to cook nutmeg jelly and nutmeg syrup, which is eaten with pancakes or used in cocktails.
Nutmeg was known in India and the Arab countries as a spice and remedy as early as 700 years B.C. It first came to Europe in the Middle Ages through Arab traders and later through Portuguese and Dutch traders.
.png)
The essential oil content of nutmegs is 5–13 %. In addition to approx. 40 % fatty oil (with the triglyceride of myristic acid as the main constituent), nutmegs also contain approx. 25 % starch and resins. The fatty oil is also known as nutmeg butter because of its buttery consistency.
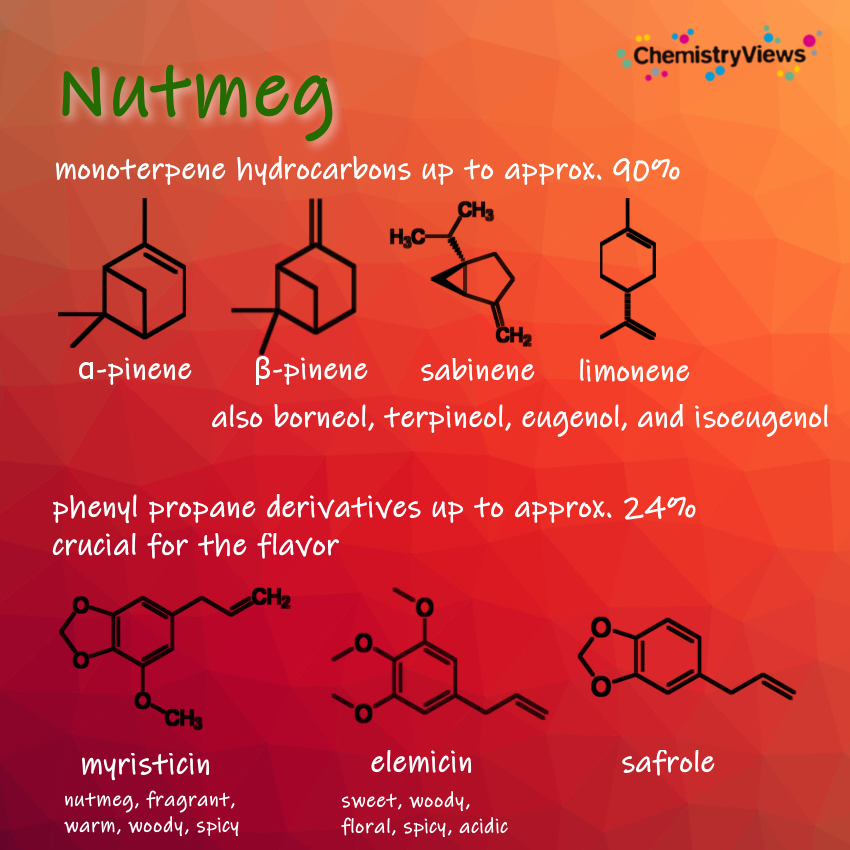
The phenylpropane derivatives such as myristicin, safrole, and elemicin act as hallucinogens. Their biological conversion products have similar structures to mescaline and amphetamines such as ecstasy (3,4-methylenedioxymethamphetamine; MDMA). Under normal use as a spice in cooking, sufficient quantities for a hallucinogenic effect are not reached. In a dose of over four grams, nutmeg is already toxic and leads to long-lasting nausea and liver damage.
References
- Dieter Abbo Kalbhen, Die Muskatnuß als Rauschdroge Ein Beitrag zur Chemie und Pharmakologie der Muskatnuß (Myristica fragrans), Angew. Chem. 1971. https://doi.org/10.1002/ange.19710831103
- Ruth Genuneit, Gewürze und Backtriebmittel in der Weihnachtsbäckerei, Institut Dr. Flad, Stuttgart, Germany, 2019. (Retrieved November 24, 2020)
- William Cook, The Physiomedical Dispensatory, 1869. (Retrieved November 24, 2020)
Also of Interest
- Chemistry Advent Calendar 2020
ChemistryViews 2020.
Daily highlights from the chemistry of spices




What happens when nutmeg oil breaks down/degrades? There is generally a soapy taste, flavour and smell. What is the chemical nature of this?