Heribert Offermanns, Merkstein, Germany, has found that chance played a role in the discovery of blue pigments, and names Berlin Blue, Cu Phthalocyanine, and YInMn Blue as examples.
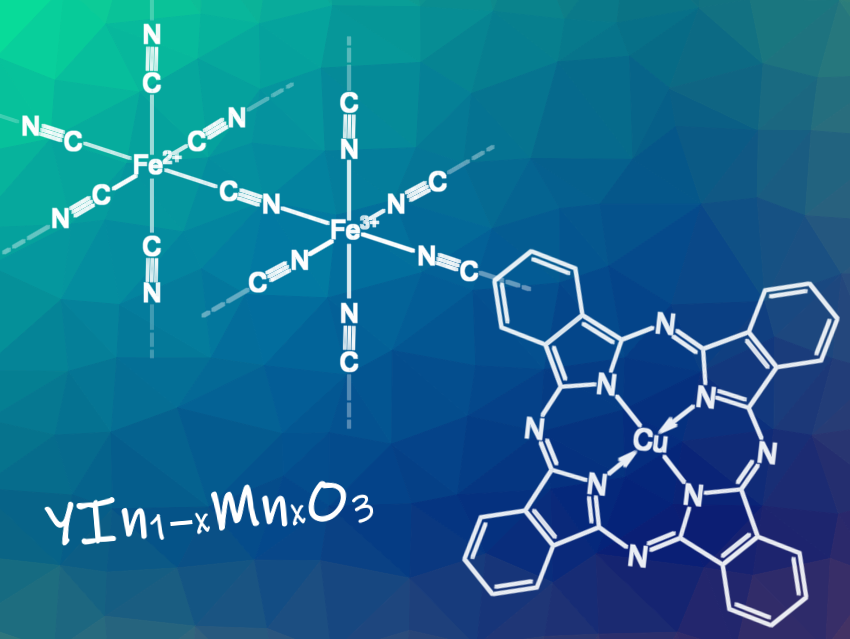
Berlin Blue (FeIII4[FeII(CN)6]3) is a lightfast, deep-blue pigment also known as Prussian Blue, Persian Blue, Turnbull’s Blue, Milori Blue, or Vossen Blue. In 1706, Johann Jakob Diesbach, Berlin, Germany, discovered it by chance: In the preparation of the red dye Florentine lacquer from cochineal lice, alum, iron(II) sulfate, and potash, he replaced the latter with Johann Konrad Dippel’s “animal oil” (or bone oil, a nitrogenous by-product of the destructive distillation of bones) and was surprised to find that he obtained a blue dye instead of a red one.
Cu Phthalocyanine Blue (C32H16CuN8) is a bright, crystalline, synthetic blue pigment, and the most important blue pigment today. In 1927, Henri de Diesbach and Edmond von der Weid, University of Fribourg, Switzerland, discovered the dye. At Imperial Chemical Industries (ICI), Grangemouth, UK, enamel flaked off during the reaction of phthalic anhydride with ammonia in an enamel-lined iron kettle. The metal became bare and a blue dye formed at this point. It was found that dyes form when phthalonitrile is reacted with heavy metals. ICI developed a manufacturing process from this, which was used for industrial production starting in 1934.
YInMn Blue (YIn1−xMnxO3) is a lightfast, non-toxic, easy-to-produce pigment also known as Oregon Blue or Mas Blue. In 2009, it was accidentally discovered by Mas Subramanian, Oregon State University, Corvallis, USA, and his then Ph.D. student Andrew E. Smith when mixing indium oxide, manganese oxide, and yttrium oxide in various ratios and heating the mixtures.
- Blaue Zufallsstunde,
Heribert Offermanns,
Nachr. Chem. 2021.
https://doi.org/10.1002/nadc.20214106887
Update (January 8, 2024): The article incorrectly stated that Henri de Diesbach and Edmond von der Weid were working in Germany in 1927. This has been corrected.



Dear Chemistry View editors,
you write in your article Blaue Zufallsstunde, Heribert Offermanns,
Nachr. Chem. 2021.https://doi.org/10.1002/nadc.20214106887 that “Cu Phthalocyanine Blue (C32H16CuN8) is a bright, crystalline, synthetic blue pigment, and the most important blue pigment today. In 1927, Henri de Diesbach and Edmond von der Weid, Germany, discovered the dye.” There is a mistake: Henri de Diesbach and Edmond von der Weid were at the University of Fribourg, Switzerland, where they discovered the dye. Please make a correction.
Thank you and best regards,
Katharina Fromm
Dear Professor Fromm,
Thank you very much for pointing this out! We have corrected the text accordingly.
Best regards,
Catharina Goedecke
Senior Associate Editor ChemistryViews.org