Photocatalytic Reduction of CO2 to Hydrocarbons
Hydrocarbons continue to be our primary source of energy. However, they do not necessarily have to come from fossil sources. Why not reverse the combustion process to make the hydrocarbons from CO2? This could be achieved by means of a solar-power-driven process if suitable catalysts were available. Researchers from Japan and China have now introduced a new, particularly efficient photocatalytic system in the journal Angewandte Chemie. It may bring us a step closer to CO2-neutral fuels.
Combining Known Catalysts
Various catalysts for the photocatalytic reduction of CO2 have previously been developed, such as those based on strontium titanate (SrTiO3, STO) or titanium dioxide (TiO2). Given the special energy levels of these two semiconductor materials, a heteromaterial made by combining the two seemed like a particularly promising approach to the research team headed by Jinhua Ye.
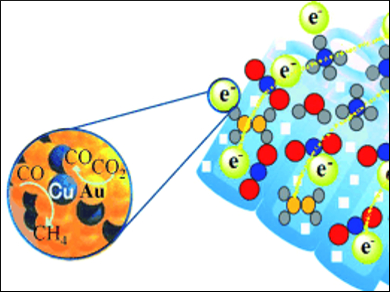
The scientists from the National Institute for Materials Science (NIMS) and TU-NIMS Joint Research Center, Tianjin University produced arrays of coaxially aligned STO/TiO2 nanotubes. They evenly loaded the nanotubes with nanoparticles of a gold-copper alloy to act as co-catalyst. Hydrazine hydrate (N2H4•H2O) acted as the source of hydrogen and maintained the necessary reducing atmosphere. This system allowed the researchers to very efficiently convert CO2 to CO and methane (CH4), as well as other hydrocarbons.
Efficient Charge Separation and Transport
Irradiation with sunlight releases electrons within the semiconductor nanotubes. The STO/TiO2 heterostructures allow the subsequent charge separation to be maintained better than in the pure substances. The electrons are transferred to the bimetallic precious metal nanoparticles and from there to the CO2, the resulting CO, and other gaseous intermediates.
The large surface area of the nanotube bundles and the porosity of the nanotube walls facilitate a high degree of gas diffusion and ensure efficient charge transport. Special effects resulting from their alloyed state allow the gold-copper nanoparticles to stop the return of photogenerated electrons in the semiconductors much more effectively than the pure metals.
The hydrazine hydrate provides the necessary hydrogen, resupplies electrons to the catalyst, and forms a reducing atmosphere, which stabilizes the metal nanoparticles for a long time. If water is used as the hydrogen source instead, the catalytic system is rapidly deactivated. On the nanoparticles, CO2 is first reduced to CO, then to CH4 and on to other hydrocarbons. A 3:1 ratio of gold to copper results in the largest amount of hydrocarbon product.
- Photocatalytic Reduction of Carbon Dioxide by Hydrous Hydrazine over Au–Cu Alloy Nanoparticles Supported on SrTiO3/TiO2 Coaxial Nanotube Arrays,
Qing Kang, Tao Wang, Peng Li, Lequan Liu, Kun Chang, Mu Li, Jinhua Ye,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201409183