CO2 – A Long-Lasting Obstacle of Solid Oxide Fuel Cells
One of the major drawbacks of carbon-fueled battery cells is the intrinsic presence of CO2, which impedes CO oxidation and, therefore, lowers the energy density of the cell. In the journal Angewandte Chemie, researchers at Nanjing Tech University and Curtin University in Perth describe a novel dual-phase ion-conducting ceramic membrane that is absolutely gas-tight but highly permeable for CO2. Integrated in a solid-oxide fuel cell (SOFC) with solid carbon as an energy carrier, it enables efficient removal of CO2 resulting in enhanced power density of the electrochemical cell.
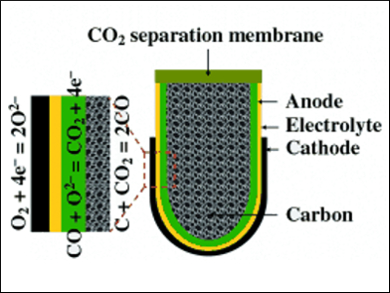
Although batteries are ubiquitously used in portable electronic devices, they are still far from being highly efficient power supplies. Recurrent issues are poor energy densities and safety, both of which particularly concern the widely used lithium-ion batteries. Prof. Zongping Shao and colleagues chose a different approach by developing electrochemical power supplies on the basis of solid-oxide fuel cells (SOFC) using solid carbon as a fuel. Their novel carbon–air battery has its fuel container – catalytically activated carbon – integrated by anode support. Its most prominent feature, however, is the addition of a ceramic membrane that has been specially designed to allow efficient CO2 separation and thus increase fuel utilization efficiency.
Efficient CO2 Removal with an Ion-Conducting Ceramic Membrane
In the battery, CO2 is formed at the electrode and then reacts with the carbon fuel to form gaseous CO, which can diffuse quickly to the electrodes and thus enhance the reaction rates. However, to obtain high energy densities, CO2 has to be separated from CO. Ceramic membranes can provide CO2 permeability through high carbonate conductivity, but, as the authors explain, it is rather the oxygen ion conducting phase, not the carbonate conductivity, which is critical for efficient CO2 permeation. Therefore, the researchers selected a samarium-containing cerium oxide (SDC) material, which is characterized by its high ionic conductivity. Sintering formed a porous SDC scaffold with fused particles to provide efficient oxygen ion conducting paths. The void spaces were then completely filled with molten carbonate to form a densified SDC–carbonate dual-phase membrane. This novel membrane not only proved to be perfectly gas-tight, but also provided a temperature-dependent excellent CO2 permeation flux much higher than the values reported for similar membranes.
“Such an improvement is likely due to the much improved densification of the membrane fabricated by using the technique reported here”, the researchers point out. Moreover, at the operating temperatures for SOFC applications, which are as high as 850 °C, attractive power densities and open-circuit voltages can be obtained. Applications may include portable energy supplies that have to be run at high temperatures.
- A Carbon–Air Battery for High Power Generation,
Binbin Yang, Ran Ran, Yijun Zhong, Chao Su, Moses O. Tadé, Zongping Shao,
Angew. Chem. Int. Ed. 2015.
DOI: 10.1002/anie.201411039