Chocolate is one of the oldest cultural assets of mankind. Its history begins with its detection by the Olmecs and reaches via the frothy cocoa beverage of the Aztecs to the modern Grand Cru chocolate. Scientists, engineers and chocolate designers have created this wonderful product from the bitter cocoa bean. The exciting chemistry involved, starting with the natural substances of the cocoa bean, continuing with the chemical changes induced by the fermenting and roasting processes, and finally reaching the stages of conching and crystallization, once again proves: Only chemistry is able to produce such celestial pleasures.
History
Cocoa was detected by the Olmecs. The members of this highly developed culture lived between 1500 and 400 BC in the tropical forests near the coasts of southern Mexico. Already at 600 BC, they consumed a beverage called kakawa [1], and they were the first to plant the cocoa tree.
Also later, in the Mayan and Aztec cultures, cocoa, here called xocoatl, played an important role, although it was only available to the rich and privileged. Cocoa was preferably consumed in frothed up form and was prepared in various ways. In addition to corn meal, flowers and diverse spices, particularly chili powder and vanilla, were added.
Many legends exist describing the first encounter of Europeans with cocoa [2]. In most cases, Hernando Cortez is brought into play, illustrating how Montezuma offered this beverage to him in a golden beaker. As a first documented proof, a present was offered by a delegation of Guatemaltecan Dominican monks and noble Mayas to their host Prince Philip of Spain in 1544. The beverage brewed from it very likely was not to Philip’s taste as it was prepared with water, without the addition of sugar, and had a bitter taste.
However, the ingenious cooks at the Spanish court performed a number of experiments with the brown beans and eventually created a tasty beverage, the recipe of which was at first kept as a secret. Only decades later, the secret mode of preparation was revealed: Raw cocoa beans were roasted above a small fire and the shells were detached from the nibs. The nibs were ground and slowly melted and then added with finely powdered sugar, the dried mark of vanilla pods, and cinnamon. “Then it has to be ground as before, but more vigorously and for a longer time, until all the ingredients are thoroughly intermingled and the whole mass looks as if it was only cocoa” [2].
Depending on the current fashion and the size of the purse, further ingredients were added to this basic recipe: musk, ambergris, jasmine flowers, spices like clove, chili pepper, black pepper, and aniseed, and also diverse nuts and even intoxicating magic mushrooms.
On this culinary basis, cocoa conquered the European nobility as it fulfilled all the prerequisites of a noble “in”-beverage: it was exotic, shockingly expensive, difficult to prepare and was told to have aphrodisiac effects.
Today, we would hardly get excited about the beverage the way it was prepared at that time. For example, the cocoa nibs were ground to a relatively coarse powder and, therefore, tasted “sandy”. The high fat content of the beans rendered it rather indigestible, and it had to be constantly frothed up to prevent both the fat and the solid compounds from settling down.
The hot beverage was proffered in a special kind of can with a lateral wooden handle, the chocolatière. Through the large opening, which could be closed with a lid, the chocolate could be stirred with a spoon to prevent the solid compounds from settling down. The servants were only allowed to prepare the expensive beverage, but not to drink it themselves.
.gif)
On the Cocoa Plantation [3]
Cocoa Tree
The cocoa tree (Theobroma cacao; food of the gods) achieves heights of up to 15 m. It grows exclusively at the equator between 15° northern latitude and 15° southern latitude, requiring sufficient humidity and temperatures that do not fall below 16 °C.
Three varieties are grown:
- Criollo is a very sensitive plant prone to infestation by diverse pests, but giving rise to high-value cocoa.
- Forastero is the most common variety, originating from the Amazon basin.
- Trinitario is a cross between the Forastero and Criollo varieties.
The total world market amounts to approximately 3.4 million tons (in 2007) per year [4].
Harvesting/Cracking
The melon-like fruit of the cocoa tree is between 10 and 30 cm in length and grows directly from the trunk and the larger branches. Apart from the flesh, it contains about 30 to 50 almond-shaped beans. This is exactly the amount required to produce one bar of chocolate.
If the untreated cocoa beans were roasted, the result would be disappointing: TAs the highly appreciated cocoa flavor only develops in fermented beans.
Immediately after harvesting, the fruits are cracked (see Fig. 1) and the beans with their flesh still attached are piled up or poured into aerated boxes, or are spread out in the open air and covered with, e.g., banana leaves.
Fermenting
After a few hours, the fermentation process sets in. In a remarkable microbiological choreography, yeast cells degrade the glucose to ethanol during the first 24 h; subsequently, lactobacilli take over and produce lactic acid. The heat released generates a temperature rise to above 37 °C, creating ideal conditions for acetic acid bacteria, which prevail after about 88 h [5]. The exothermic oxidation of alcohol and lactic acid to acetic acid leads to a rise in temperature to above 50 °C and a decrease in pH to values of <5. Thereby, all fermentation processes and the germination of the beans are stopped.
The course of this fermentation phase can be followed externally, by monitoring the initially increasing, then decreasing acetic acid flavor.
During fermentation, preliminary flavor compounds for the later roasting step are formed. Two protein-degrading enzymes (asparagine protease and a serine protease) successively decompose a cocoa storage protein, vicilin (7S)-class globulin [6, 7]. In the following roasting step, the resulting oligopeptides react with sugars to form the characteristic flavor compounds.
Drying
After several days of fermentation, the beans are spread out on mats and dried in the sun for one to two weeks. During drying, the beans lose some moisture and the greater part of their acetic and lactic acid contents. After drying, the beans are ready for shipment, but because of their bitterness, they are still inedible.
In the Chocolate Factory
Roasting
Following delivery and quality control, the beans are roasted for 1–2 h. At 100–120 °C, the fat in the beans melts and in this “solvent” innumerable compounds react, catalytically enhanced by metal ions and the polysaccharides of the cell walls. The degradation of polyphenolic tannins is important for sensory characteristics as thereby the sour and the astringent taste are ameliorated.
During roasting, water and several unattractively smelling chemical compounds containing acetic, propionic, and iso-butyric acid are evaporated.
However, the real chemical marvel of roasting is the metamorphosis of the musty smelling cocoa beans into the seductively nice-smelling, dark brown ones. Under its harsh conditions, amino acids and sugars react in a non-enzymatic browning reaction, the Maillard reaction [8]. The chemical processes are extremely complex as many reaction cascades occur in parallel. The resulting intermediate and end products are highly reactive and, therefore, react with themselves and with each other. In the course of these reactions, all the flavor compounds shown in Fig. 2 are formed, as well as the polymeric dark brown chemical compounds that give the roasted cocoa bean its unique flavor and its typical color.
.gif)
The Maillard reaction can also take place under milder and controlled conditions. For example, already during heating of an aqueous solution of glucose and an amino acid like threonine, leucine or glutamine, flavor compounds that begin to smell like chocolate are formed [9, 10].
Grinding
.gif)
After roasting, the beans are broken into larger pieces and the shells are removed. Using mills and rollers, the up to now still intact cell structures of the cocoa bean nibs are destroyed and the fat contained therein is released. After thorough and multi-step rolling, the basis of all cocoa and chocolate products is eventually formed: the cocoa paste (see Tab. 1).
This paste smells and tastes like chocolate; however, in order to arrive at the crisp and tasty chocolate bar, there is still a long process ahead. A process that has, in the course of many years, been discovered through the congenial interaction of engineers, chemists, and chocolatiers.
Pressing
The year 1828 marks the beginning of the modern chocolate production, when the Dutch chemist Coenraad Johannes van Houten patented a method to partially squeeze the fat from cocoa paste batches (fat content of > 50 %). The squeezed out cocoa fat, called cocoa butter, is then separated and the residual brown press cake (fat content of 20 %) is ground to today’s well-known cocoa powder (see Fig. 1).
Van Houten had another brilliant idea: Before and after roasting, the cocoa beans can be treated with aqueous lye (“Dutching”). The increase in pH value from about 5.4 to 7.0 results in a milder tasting, darker cocoa powder that is easy to disperse (but not to dissolve!) in water or milk. So, do not be alarmed if on today’s cocoa powder packages the acidity regulators potassium carbonate and sodium hydroxide can be found.
At the time of van Houten, only the cocoa powder was of interest; the squeezed out cocoa butter was only a waste product. In 1847, the English company Fry&Sons created a solid chocolate for eating from cocoa paste, sugar and, in addition, cocoa butter. The new recipe for the first time allowed the pouring of very thin chocolate bars with an elegant flair. This “Chocolat Délicieux à Manger” was the first modern chocolate bar, and was an immediate and great success. As higher amounts of cocoa butter than of cocoa paste were required for its production, the price for cocoa butter shot up. And this persists until now: Cocoa butter is the most expensive vegetable fat.
On the contrary, cocoa powder became cheap and generally affordable. Milk chocolate or hot chocolate became popular beverages!
Conching
We must be grateful to the Swiss chocolatier Rudolphe (Rudi) Lindt (1855–1909) for a step in quality improvement that cannot be overestimated. He constructed a conch-shaped trough made of granite, the conche. In this trough, the cocoa paste together with other ingredients like sugar and cocoa butter is moved to and fro by granite rollers (see Fig. 3). The frictional heat induces the chocolate paste to melt, and when the roller hits the rim of the vessel, the liquid mass splatters back into the vessel.
.jpg)
At first glance, conching seems to be just another mechanical treatment step, in which the solid cocoa bean particles dispersed in the cocoa butter are ground to a size below 20 µm and are simultaneously coated with a layer of cocoa butter. However, thereby our tongues no longer perceive the solid particles as such, or, in other words: before Lindt, the chocolate tasted sandy, but after conching it melted on the tongue like soft cream.
Conching not only improved the mouth feel but also the flavor. From the chemical point of view, this seems at first not plausible. However, at a closer look, it becomes obvious: Through the crushing of the solid cocoa particles, the surface increases. Thereby, the flavor compounds enclosed in the particles can diffuse into the surrounding fat and the flavor becomes more intensive.
Eventually, the mechanical aeration of the warm liquid chocolate paste expels the volatile compounds with undesired flavor characteristics such as acetic, propionic and iso-butyric acid, together with the residual moisture, by a kind of water vapor distillation.
Overall, the treatment of the chocolate mass in the conche has major effects on the flavor development and the consistency of the chocolate. Without conching, there would be no high-value chocolate and, for each chocolate producer, the conche is still the core element (see Fig. 3). In today’s industrial chocolate production, conching machines with rotating scoops are used. Smaller-scale producers of high-value chocolate, however, still use the traditional conche according to Lindt.
Conching is performed in three steps, and in each phase, the gradual grinding of the solid compounds and the rising degree of mixing and coating with cocoa butter leads to a further reduction in viscosity.
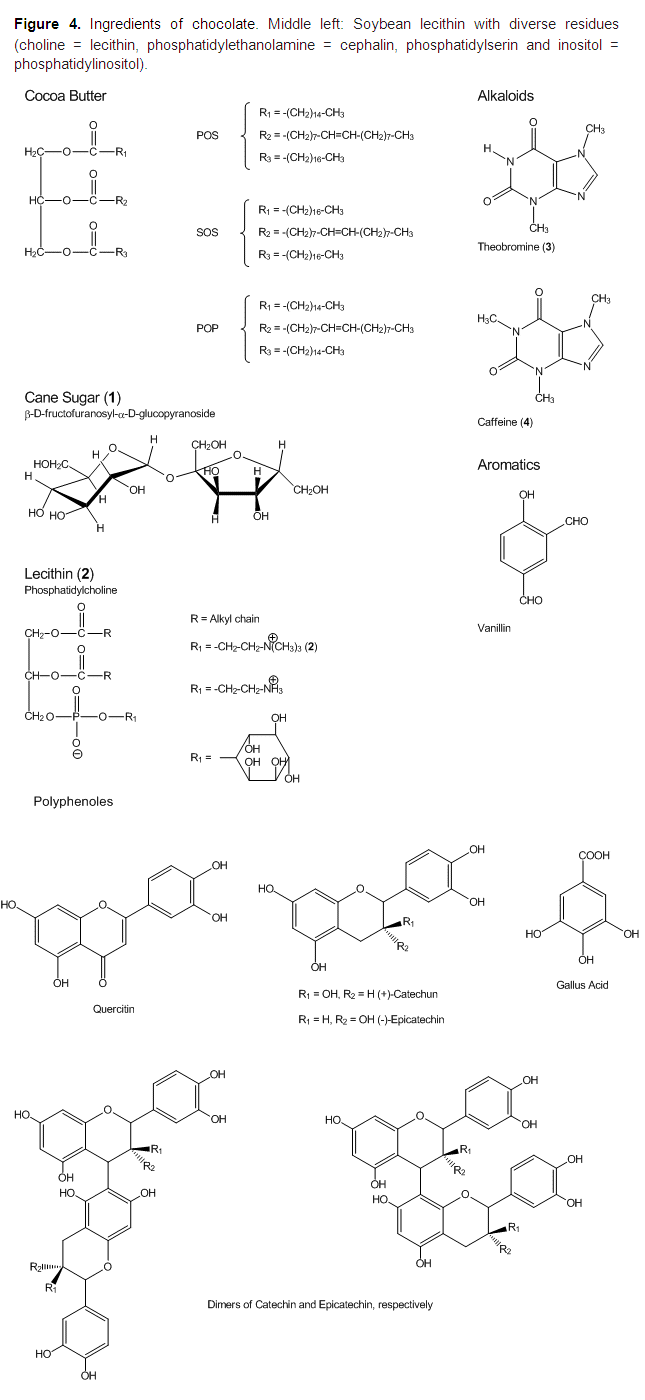
Lecitin As An Emulsifier
Since the 1930s, the chocolate paste is supplemented during the conching process with lecithin as an emulsifyer. Chemically, lecithin is identical to phosphatidylcholine (2), which occurs, e.g., in sunflower seeds, rapeseed, corn or egg yolk. However, in the field of food technology, lecithin is not defined as pure (2), but as a mixture of diverse phospholipids, (additive E 322) (see Fig. 4). In chocolate, the lecithin from soybeans is nearly exclusively utilized.
The addition of only 0.1–0.3 % lecithin already reduces the viscosity of the molten chocolate paste to one-tenth of its original value. A comparison between the structures of lecithin and the two main components of chocolate, cocoa butter and sugar (see Tab. 2), explains the cause of this effect:
.gif)
Cocoa butter is a typical fat: The three hydroxyl groups of the trivalent alcohol glycerol are esterified with the three most common long-chain fatty acids: palmitic acid (P) CH3(CH2)14COOH, stearic acid (S) CH3(CH2)16COOH, and oleic acid (O) CH3(CH2)7CH=CH(CH2)7COOH.
While the fatty acids of most fats are equally distributed over the three positions of glycerol, and thus generate a mixture of many different molecules, cocoa butter consists of only three different compounds (40 % POS, 25 % SOS, and 20 % POP). Oleic acid is always situated at the middle 2-position, and palmitic and stearic acid are bound to the 1- and 3-positions of glycerol (see Fig. 4)..gif)
The sugar in chocolate is cane sugar (sucrose). Due to the hydroxyl groups, cane sugar is highly polar and easily soluble in water, but nearly insoluble in lipids.
All components of lecithin are constructed in the same way: Glycerol is esterified with two nonpolar (lipophilic) fatty acids and one polar, charged (hydrophilic) phosphate group attached to a variable residue.
After addition of lecithin to the chocolate paste, its polar groups bind to the polar hydroxyl groups on the surface of the sugar crystal, so that the crystal is wrapped in a closed lecithin layer. The two nonpolar fatty acid residues of lecithin bind to the nonpolar fatty acid residues of the surrounding cocoa fat (see Fig. 5).
Thereby, through the intermediate layer of lecithin molecules, the polar and lipid-insoluble small sugar crystals are much better emulsified in the fat. And thus, the viscosity decreases.
Prof. Klaus Roth,
Freie Universität Berlin, Germany
The article has been published in German in:
and was translated by Monika Kortenjann.
References
[1] W. J. Hurst et al., Nature 2002, 418, 289. DOI: 10.1038/418289a
[2] S. Coe, M. D. Coe, The True Histrory of Chocolate, Thames and Hudson, London 1996.
[3] www.quarks.de/schokolade/
[4] www.icco.org
[5] R. F. Schwan, A. E. Wheals, Crit. Rev.Food Sci. Nutr. 2004, 44, 205. PMID: 15462126.
[6] B. Biehl et al., Food Chem. 1994, 49, 173. DOI: 10.1016/0308-8146(95)00188-3
[7] B. Biehl et al., J. Sci. Food Agric. 2002, 82, 728. DOI: 10.1002/jsfa.1104
[8] M. Angrick, D. Rewicki, Chem. unserer Zeit, 1980, 14, 149. DOI: 10.1002/ciuz.19800140503
[9] G. Tannenbaum, J. Chem. Educ. 2004, 81, 1131. DOI: 10.1021/ed081p1131
[10] G. Tannenbaum, Lessons in Chocolate, Flinn Scientific Inc., Batavia, USA 1993.
[11] S. Schenker, Nutr. Bull. 2000, 25, 303. DOI: 10.1046/j.1467-3010.2000.00075.x
Part II
Chocolate – The Noblest Polymorphism
Klaus Roth describes why chocolate tastes so good: it is a unique substance, solid at room temperature, and liquid at body temperature. Cocoa butter can crystallize into six polymorphic forms, but only one of them shows the noble surface sheen, the crisp hardness, and the pleasant melting in the mouth.
Other articles by Klaus Roth published by ChemViews magazine:
-
- In Espresso — A Three-Step Preparation
Klaus Roth proves that no culinary masterpiece can be achieved without a basic knowledge of chemistry
DOI: 10.1002/chemv.201000003 - In Chemistry of a Hangover — Alcohol and its Consequences
Klaus Roth asks how a tiny molecule like ethanol can be at the root of so much human misery?
DOI: 10.1002/chemv.201000074 - In Sparkling Wine, Champagne & Co
Klaus Roth shows that only chemistry can be this tingling
DOI: 10.1002/chemv.201000047 - In The Chemist’s Fear of the Fugu
Klaus Roth shows the chemist’s fear of the fugu or pufferfish extends as far as the distinctive and intriguing poision it carries
DOI: 10.1002/chemv.201000104
- In Espresso — A Three-Step Preparation





I am actually thankful to the owner of this website who has shared this wonderful post here.
Thank you! We’re glad to hear that and also truly grateful to the author of this article.
I am happy to meet this article again after 20 years. Enjoy it while sprawling on the couch nibbling fine chocolate!