Syngas is a mixture of H2 and CO. It is widely used, e.g., in methanol synthesis or olefin hydroformulaton. CO2 is a suitable feedstock for syngas production, as it is easily available, affordable, and can be captured from power stations. Hydrogen or methane are possible co-reactants to convert CO2. To obtain a carbon saving potential, aditional CO2 emission has to be avoided during production of H2 and CH4.
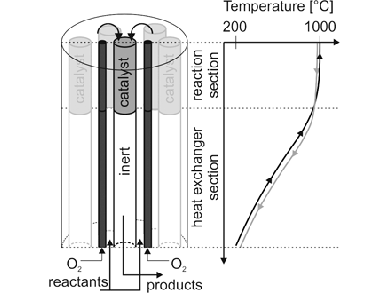
H2 can be produced from electrolysis of water with renewable energy, whereby pure oxygen is obtained. The oxygen can be used for simultaneous partial combustion of the energy-rich co-reactants H2 or CH4 to obtain high temperatures. This is crucial for both, high CO2 conversion and to avoid coke formation. René Kelling, Gerhart Eigenberger, and colleagues, University of Stuttgart, Germany, have developed a scalable reactor concept for these harsh reaction conditions. Their multitubular reactor (pictured) consists of ceramic reaction tubes partially coated with a reforming catalyst.
An optimal oxygen flow profile and efficient heat-recovery ensure constant temperatures around 1000 °C in the reaction section. More than 85 vol % of hydrogen and CO are produced with CO2 conversion of around 70 %. A model predicts high CO2 conversion and syngas production in industrial scale while maintaining defined temperature limits so that coke formation is avoided.
The researchers think that their reactor concept is applicable also for other high temperature synthesis heated by simultaneous combustion such as autothermal steam reforming or partial oxidation of methane without CO2.
- Ceramic Counterflow Reactor for Efficient Conversion of CO2 to Carbon-Rich Syngas,
René Kelling, Hendrik Dubbe, Gerhart Eigenberger, Ulrich Nieken,
Chem. Ing. Tech. 2015.
DOI: 10.1002/cite.201400128