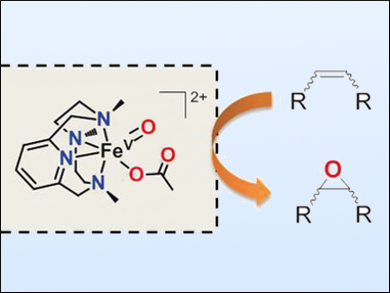
Oxygen-atom transfer to alkanes and alkenes is a valuable reaction in organic synthesis. It can produce alcohols or epoxides, respectively. The use of nonheme iron complexes as catalysts, combined with hydrogen peroxide, is emerging as a particularly efficient biologically inspired strategy. However, elucidation of the mechanistic details of these reactions is difficult because the active species are very reactive, and most often do not accumulate in solution, thus their direct detection has remained a challenge.
Anna Company, Miquel Costas, University of Girona, Spain, Manuel G. Basallote, University of Cádiz, Spain, and colleagues have successfully trapped a highly reactive metastable nonheme iron compound. It exists as a pair of electromeric species, [FeIII(OOAc)(PyNMe3)]2+ and [FeV(O)(OAc)(PyNMe3)]2+ (pictured), in fast equilibrium. The team used stopped-flow UV/Vis analysis and found that oxygen atom transfer from these species to alkenes is exceedingly fast.
This complex is capable of performing stereoretentive alkane hydroxylation and olefin epoxidation, with unprecedented reaction rates and high selectivities. Furthermore, the observed correlation between reaction rates and selectivity properties shows that this species is a promising oxygen atom transfer agent with possible applications in biologically inspired nonheme iron oxidation catalysis.
- Exceedingly Fast Oxygen Atom Transfer to Olefins via a Catalytically Competent Nonheme Iron Species,
Joan Serrano-Plana, Almudena Aguinaco, Raquel Belda, Enrique García-España, Manuel G. Basallote, Anna Company, Miquel Costas,
Angew. Chem. Int. Ed. 2016, 55, 6310–6314.
DOI: 10.1002/anie.201601396
Also of Interest
- Clara Immerwahr Award 2015,
ChemViews Mag. 2015.
Anna Company Casadevall, University of Girona, Spain, honored