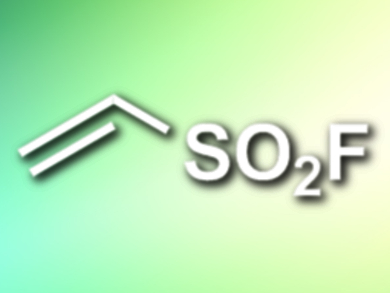
Ethenesulfonyl fluoride (ESF) is a highly reactive Michael acceptor and has applications, e.g., in the productions of dyes, functional materials, and pharmaceuticals. However, the compound’s high price limits its potential for wider use.
K. Barry Sharpless, Scripps Research Institute, La Jolla, CA, USA, and colleagues have developed a simple kilogram-scale synthesis of ESF. The team started from the readily available 2-chloroethanesulfonyl chloride, which was added to an aqueous solution of potassium bifluoride, K(FHF). The system forms two phases, which were stirred to form an emulsion for two hours, during which the 2-chloroethanesulfonyl chloride was converted to 2-chloroethanesulfonyl fluoride. The product could be isolated easily using a separatory funnel with a yield of more than 99 %.
The obtained 2-chloroethanesulfonyl fluoride was converted into ESF in a similar manner by adding it to water, stirring to form an emulsion, and adding magnesium oxide to induce a dehydrochlorination. Again, the product could simply be isolated using a separatory funnel. The reaction produces ESF with a yield of over 98 % on a kilogram scale. According to the researchers, this straightforward approach should also be practical on a commercial scale.
- Ethenesulfonyl Fluoride (ESF): An On-Water Procedure for the Kilogram-Scale Preparation,
Qinheng Zheng, Jiajia Dong, K. Barry Sharpless,
J. Org. Chem. 2016.
DOI: 10.1021/acs.joc.6b01423
Also of Interest
- Video: How Discoveries Are Made,
Vera Koester,
ChemistryViews.org 2016.
DOI: 10.1002/chemv.201600039
K. Barry Sharpless, 2001 Chemistry Nobel Laureate and Scripps Research Institute, La Jolla, CA, USA, explains how scientific discoveries are made