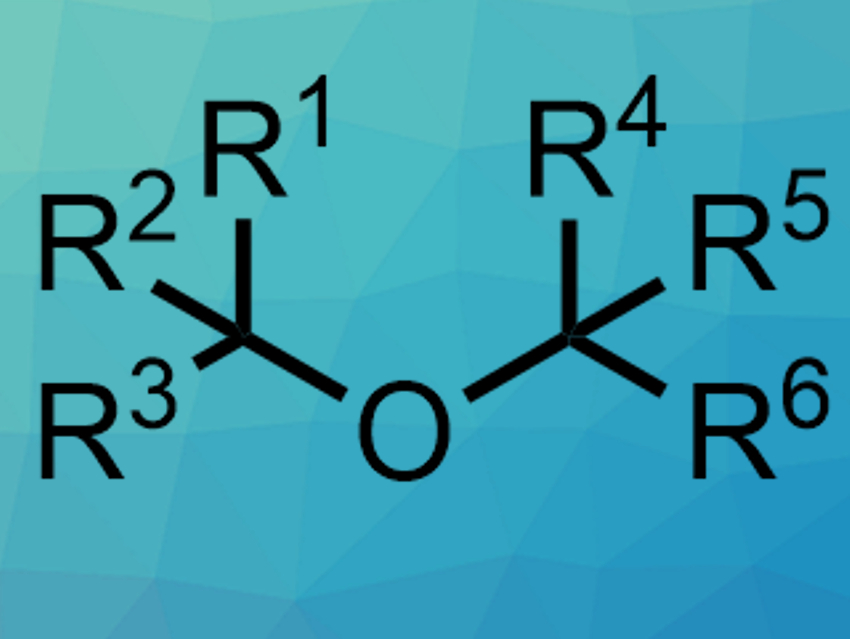
Ethers with many bulky substituents, or hindered ethers (pictured), can be challenging to synthesize. Donna G. Blackmond, Phil S. Baran, The Scripps Research Institute, La Jolla, CA, USA, and colleagues have developed a method for the synthesis of hindered dialkyl ethers, based on an electrochemical approach.
The team used an electrochemical oxidation to generate carbocations from simple carboxylic acids, with a loss of CO2. These carbocations can be captured by an alcohol to give an ether. The researchers optimized the reaction conditions. They found that the best results were obtained with inexpensive graphite electrodes and in the presence of 2,4,6-collidine as a base, n-Bu4NPF6 as the electrolyte, AgPF6 as an additive, and 3 Å molecular sieves, with dichloromethane (DCM) as the solvent.
Using this method, they prepared a variety of ethers (over 80 examples) that would be difficult to access with other methods. The team also used the approach to improve the efficiency of several particularly challenging syntheses of hindered ethers. These ethers could be useful for pharmaceutical or materials applications. The team was able to reduce the number of steps (from 6.3 to 1.5 on average) and improve the yields of these syntheses (from 19 % to 43 % on average) compared to previously used approaches.
- Hindered Dialkyl Ether Synthesis via Electrogenerated Carbocations,
Jinbao Xiang, Ming Shang, Yu Kawamata, Helena Lundberg, Solomon Reisberg, Miao Chen, Pavel Mykhailiuk, Gregory Beutner, Michael Collins, Alyn Davies, Matthew Del Bel, Gary Gallego, Jillian Spangler, Jeremy T. Starr, Shouliang Yang, Donna Blackmond, Phil Baran,
ChemRxiv 2019.
https://doi.org/10.26434/chemrxiv.8144294.v1The research has been published as a preprint and has not yet been peer-reviewed.
Also of Interest
- Video: Phil Baran on What Makes a Good Chemist,
ChemistryViews.org 2017.
Phil Baran about his research and what makes a good chemist - Interview: The Charm and Appeal of Organic Chemistry,
Phil S. Baran, Frauke Zbikowski,
ChemViews Mag. 2017.
https://doi.org/
Interview with Phil Baran, who is well known for the synthesis of complex molecules - Dawn of a New Age in Synthetic Organic Electrochemistry,
ChemViews Mag. 2017.
https://doi.org/10.1002/chemv.201700062
Phil S. Baran presented the ElectraSyn 2.0 and demonstrated how easy organic reactions will become