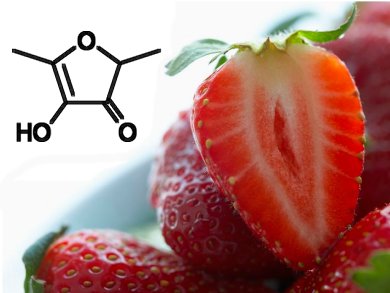
Fresh strawberries owe their typical aroma to the volatile compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone (HDMF, pictured). During ripening, the NAD(P)H dependent enzyme Fragaria x ananassa enone oxidoreductase (FaEO) synthesizes HDMF by reducing an exocyclic double bond present in 4-hydroxy-5-methyl-2-methylene-3(2H)-furanone (HMMF).
The exact mechanisms of this reaction have been elucidated by André Schiefner and colleagues, Technische Universität München, Germany. The scientists revealed that FaEO transfers a hydride ion from NAD(P)H to HMMF’s unsaturated exocyclic C6 carbon. In doing so, FaEO generates a cyclic non-chiral enolate intermediate which is converted into HDMF following protonation. By elucidating the structural basis that regulates HDMF synthesis, this study offers new insights to improve the production of a volatile largely used in the food and perfume industries.
- Structural basis for the enzymatic formation of the key strawberry flavor compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone,
A. Schiefner, Q. Sinz, I. Neumaier, W. Schwab, A. Skerra,
J. Bio. Chem. 2013.
DOI: 10.1074/jbc.M113.453852