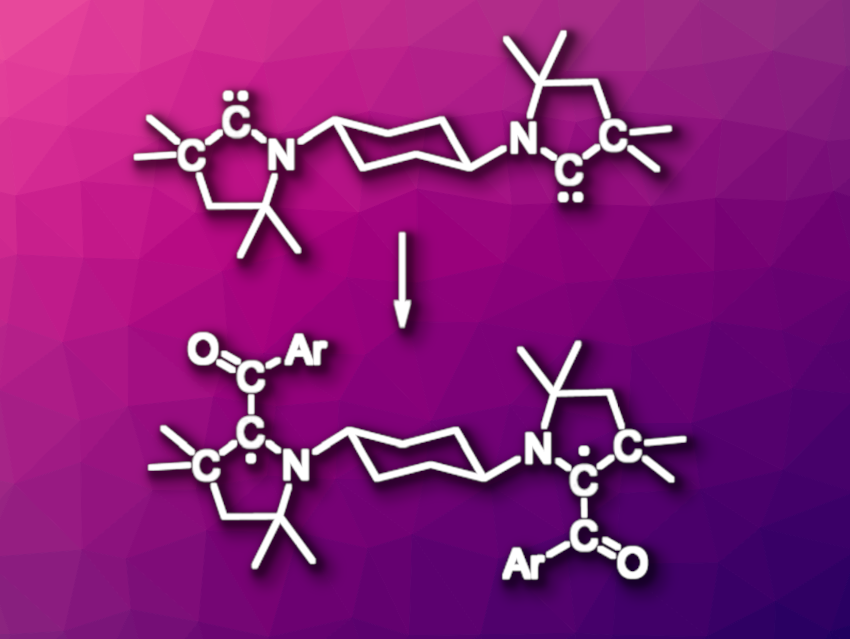
Cyclic(alkyl)(amino)carbenes (CAACs) can be useful electron-rich alternatives to the commonly used N-heterocyclic carbenes (NHCs). They have applications, e.g., as ligands in catalysis. The structure of CAACs is also suitable for stabilizing radicals centered at the carbene carbon atom. Often, such radicals are prepared from the carbene via a pyrrolinium cation intermediate, which is then reduced. Diradical species can be realized by connecting two CAAC units using a suitable linker.
Abhishake Mondal, Indian Institute of Science, Bangalore, India, Holger Braunschweig, Prince Ravat, University of Würzburg, Germany, Carola Schulzke, University of Greifswald, Germany, Anukul Jana, Tata Institute of Fundamental Research, Hyderabad, India, and colleagues have developed an approach to obtaining bis-CAAC-based diradicals (example pictured in the lower row) from bis-CAACs. The team first synthesized bis-CAACs (example pictured in the top row) that are connected via a trans-1,4-cyclohexylene linker bound to the two nitrogen atoms. The bis-CAACs were obtained in the form of their LiOTf adducts by the deprotonation of the corresponding bis-pyrrolinium dications using lithium tetramethylpiperidide (LiTMP).
The resulting bis-CAAC adducts were reacted with 2-naphthoyl chloride to form dicationic intermediates. The researchers reduced these intermediates using tetrakis(dimethylamino)ethylene (TDAE) and obtained the desired diradical species. The products are crystalline and thermally stable. They were characterized using, e.g., UV/Vis spectroscopy, single-crystal X-ray diffraction, and electron paramagnetic resonance (EPR) spectroscopy. The latter showed a triplet character of the diradicals. According to the team, the developed synthetic route could be useful for the preparation of a wider scope of bis-CAAC-based diradical species.
- Bis-[cyclic(alkyl)(amino)carbene]-derived diradicals,
Mithilesh Kumar Nayak, Benedict J. Elvers, Sakshi Mehta, Ivo Krummenacher, Abhishake Mondal, Holger Braunschweig, Carola Schulzke, Prince Ravat, Anukul Jana,
Chem. Commun. 2024.
https://doi.org/10.1039/D3CC05779H
Also of Interest
-
Dicationic Diradical Based on Carbene Dimers,
ChemistryViews 2022.
Two dimers based on N-heterocyclic carbenes (NHCs) and cyclic(alkyl)(amino)carbenes (CAACs) bridged by a cyclohexyl unit