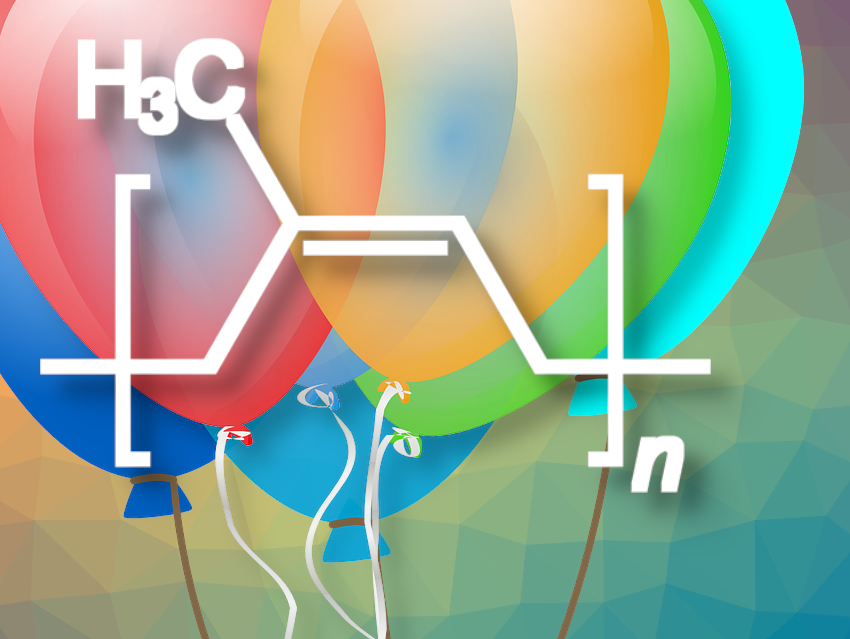Balloons transport us into many a childhood dreamland—and come from the Hevea brasiliensis plant. The transformation of a creamy, white natural substance into a colorful balloon requires a helping hand from the gods, and only Vulcan—the Roman god of fire and a gifted chemist—can perform such a miracle. We have not yet seen through all his tricks, but we will try to follow his chemical trail.
In this part, we will look at the discovery of vulcanization. Was it a happy accident or the result of hard work?
3 Vulcanization: An Alchemical Marvel with a Touch of the Divine
We should not let the applications devised for rubber before the beginning of the 19th century, such as raincoats and erasers, fool us into thinking that broader technical applications of natural rubber were inconceivable. Natural rubber became truly significant when Charles Goodyear discovered vulcanization in 1839.
3.1 Discovery of Vulcanization
Charles Goodyear
The discovery of vulcanization is usually described as a lucky accident. Charles Goodyear (see Fig. 5) [7] is said to have spilled a mixture of rubber, sulfur, and lead oxide on a hot stove, resulting in a highly elastic mass—much to his surprise. Humorist Bill Bryson quipped, “Goodyear personified most of the qualities of the classic American inventor—total belief in the product, years of sacrifice, blind devotion to an idea—but with one engaging difference. He didn’t have the faintest idea what he was doing.”
This is grossly unfair to Goodyear, because in reality he had—obsessed with the idea of making a useful material out of raw rubber—unsuccessfully tried out thousands of different formulations over many years. He was constantly in heavy debt, and his family financed his research projects at great personal sacrifice. It is true that the discovery is not the result of a scientific study, but sometimes trying things beats hitting the books. The discovery was granted to him as a reward for his talent in capitalizing on his luck at the right moment.

Figure 5. Charles Goodyear.
Thomas Hancock
Because Goodyear did not have enough money to patent and market his process, he looked for people to invest in his “tanned” rubber, sending several samples to English industrialists. In 1842, Thomas Hancock got his hands on one of these samples. Hancock was a successful manufacturer who had been using an entirely novel technology to produce elastic threads and rubber bands since 1820.
In an attempt to process scraps of rubber, he discovered that mechanical shredding and heating could be used to convert the scraps to a homogenous and formable rubber mass. In his new device, called a masticator, the strong mechanical forces radically break up the polymer molecules. The reduction of the average molecular weight greatly increases the processability of the material.
Hancock recognized the significance of his development, but did not patent it, operating his machines in secret. To mislead the competition, he referred to his more easily processable rubber as “pickled”.
Patent Dispute
The sample he was sent by Charles Goodyear could have allowed Hancock to detect the high sulfur content from the smell or by burning, making it possible for him to figure out Goodyear’s trick, but he was not able to do it. Hancock developed an alternative process in which rubber strips were dipped in molten sulfur. At the suggestion of a friend, he called the process “vulcanization” after the Roman god of fire. He wanted to reflect the fact that the chemical process involved sulfur and high temperatures, both attributes of active volcanoes—home of Vulcan, the Roman god of fire.
Hancock was a clever businessman and patented his vulcanization process on November 21, 1843, eight weeks before Charles Goodyear (January 30, 1844). Endless and unpleasant patent suits were filed by Goodyear and Hancock, as well as other competitors. From our perspective today, we can say that they both, Goodyear as a tinkerer and Hancock as an engineer and industrialist, laid the foundation for this industry, which still flourishes today [8].
The modern company known as the Goodyear Tire & Rubber Co. was founded in 1898, almost 40 years after Goodyear’s death, by the Sieberling brothers in Akron, Ohio, USA. The Goodyear name was used for marketing purposes; Charles Goodyear and his descendants were not involved in any way.
3.2 Vulcanization and Elasticity and Thermoplasticity
In natural rubber, the polyisoprene chains are tightly tangled up with each other. The individual chains, which are not chemically bound to each other, can easily slide past each other, especially at elevated temperatures.
This is why an external pulling force exerted on a sample of natural rubber leads to a plastic deformation. After the pulling force is removed, the sample does not fully regain its former shape, because the polyisoprene chains are now in an arrangement of nearly equal energetic favorability to the original one. Natural rubber is thus thermoplastic, but not very elastic.
In the vulcanization process, the individual chains are chemically attached to each other by sulfur bridges, forming a network. If an identical external pulling force is applied to a sample of vulcanized rubber, only minor deformation occurs, because the sulfur bridges prevent the polyisoprene chains from sliding apart. When the pulling force is removed, the sample returns to its original shape. Vulcanized rubber is elastic, but not very thermoplastic.
3.3 The Chemistry of Vulcanization
Naming the process after a god was truly justified, since it remains an almost supernatural wonder to this day. The chemistry is complex because the reaction conditions are extreme, and many parameters play a role. Ultimately, some of the double bonds are attacked by sulfur (see Fig. 6), resulting in the formation of polysulfide bridges between the isoprene chains. This forms a cross-linked, three-dimensional macromolecule.
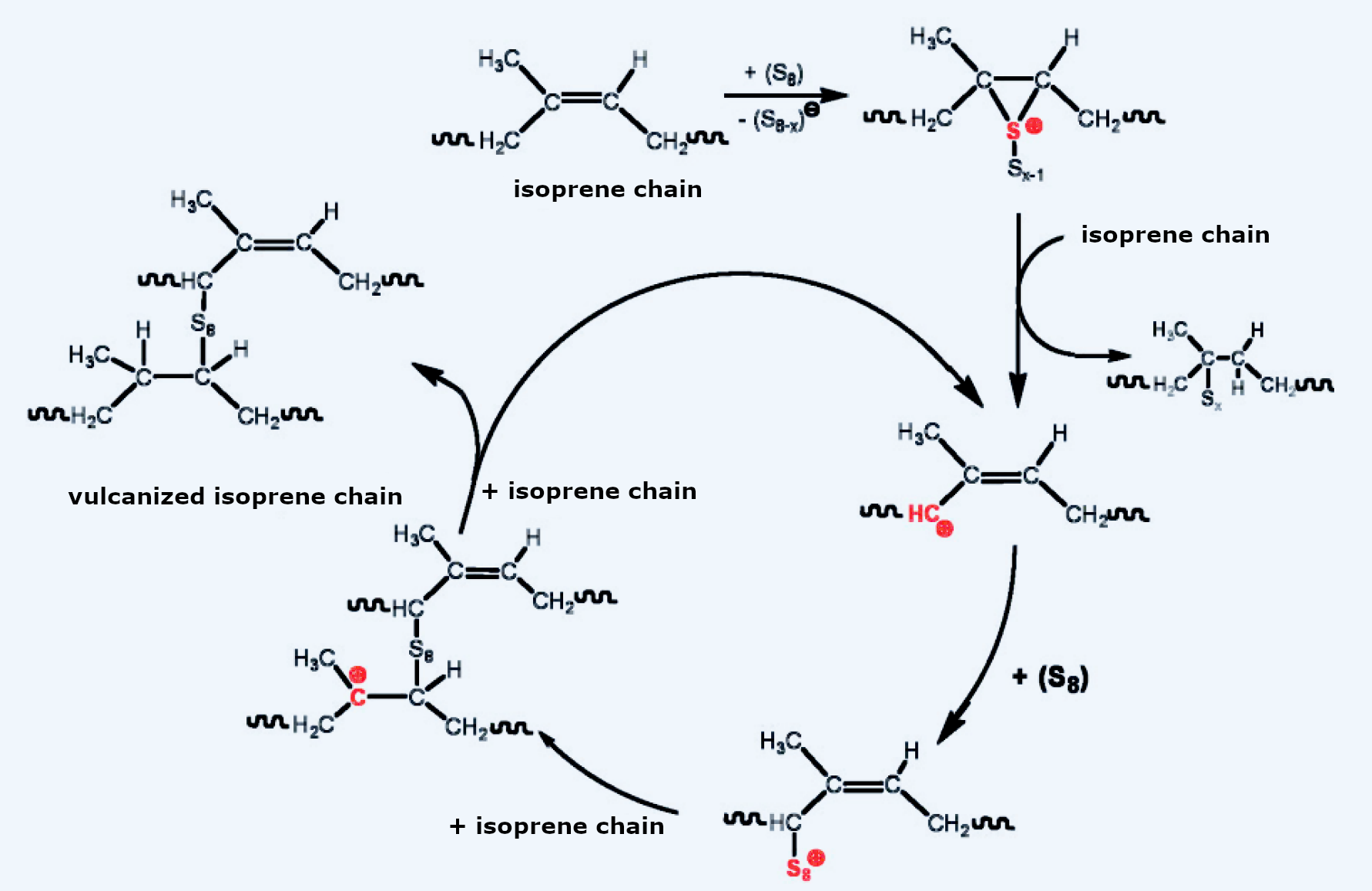
Figure 6. The chemistry of vulcanization.
Because the rate of vulcanization is accelerated by salts instead of radical formation, it can be assumed that the mechanism is ionic. According to Hans-Georg Elias [9], an S8 ring attacks a double bond, splitting off a polysulfide anion. The resulting cation reacts with a second isoprene unit, forming an addition product and a carbocation. This carbocation initiates the actual reaction cycle: it reacts with another S8 ring, and the resulting cation electrophilically attacks a second isoprene unit. Reaction with another isoprene forms the product—vulcanized by an S8 chain—and a carbocation, which begins the reaction again.
3.4 Improvements to the Vulcanization Process
Since Goodyear’s discovery, generations of scientists have improved the vulcanization process. Today, rubber is heated together with a variety of additives in addition to sulfur. These additives only make up a small percentage of the weight, but they decidedly improve the quality of balloons:
Sulfur
The number of cross-linking sulfur bridges determines the hardness of the product. Sulfur content above 35 % results in highly cross-linked hard rubber, less than 5 % sulfur content forms soft rubber. In the vulcanized rubber used in balloons, the sulfur content is kept under 1 % to maintain high elasticity so that children can blow them up. This means that only about one in 100 monomer units are cross-linked.
Catalysts and Activators
Sulfur is relatively inert, so vulcanization is a slow process even at high temperatures. Under these conditions, the long isoprene chains are partially degraded. This heat damage leads to inadequate strength and low resistance to aging. Catalysts make it possible to lower the temperature and time needed for vulcanization, as well as reducing the amount of sulfur required.
The decreased thermal stress also makes it possible to add organic pigments, which allows for production of a rainbow of colors.
Many of the catalysts used industrially give vulcanization products a bitter taste and cannot be used in products that come into contact with foods. For balloons, flavorless zinc dialkyldithiocarbamate (3) has been established as reliable (see Fig. 7). This compound easily reacts with elemental sulfur to form polysulfide R2N-C(S)-S-(Sn)-Zn-S-C(S)-NR2. Its catalytic effect is based primarily on the higher reactivity of this compound relative to elemental sulfur.
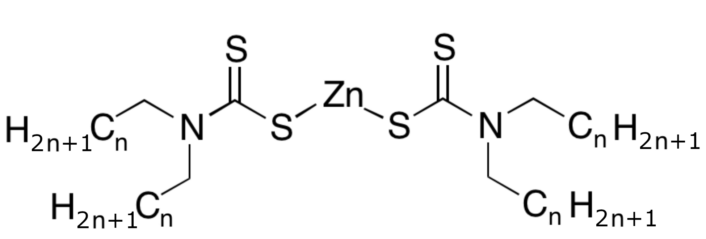
Figure 7. Zinc N,N-dialkyldithiocarbamate (3).
To fully optimize the catalysts, activators are added. These do not directly speed up the vulcanization; they increase the efficiency of the catalyst. Zinc oxide (3–5 wt%) has proven to be a particularly good activator and is found in many rubber products. The activation effect of zinc oxide results from the formation of S-Zn-S bonds.
Finally, fatty acids are also added. These independently further activate the entire rubber-sulfur-catalyst-zinc oxide system. Here we must acknowledge the ingenuity of industrial chemists. To put it precisely, they have succeeded in having the activators catalyze the catalysts and the fatty acids catalyze the catalysts of the catalysts.
Age Resistance
After use or storage, elastic materials lose their elasticity and tensile strength. Various factors influence this ageing process, such as mechanical strain, heat exposure, and oxidation reactions with oxygen and ozone. Addition of free radical scavengers can significantly slow these ageing processes.
After a week in a pure oxygen atmosphere (21 bar, 70 °C), a vulcanized rubberband without age protection retains only 20 % of its original tensile strength; after addition of 2 wt% of age protection it is still 85 % as strong as at the start. The age protection used in this experiment was a derivative of 1,4-diaminobenzene that is not approved for use in balloons [10]. Only a few antioxidants, such as the phenol derivative (4), are approved for use in balloons, which people put in their mouths (see Fig. 8).
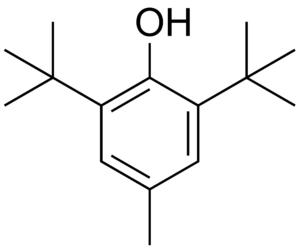
Figure 8. 2,6-Di-tert-butyl-4-methylphenol (4).
References
[7] Richard Korman, The Goodyear Story: An Inventor’s Obsession and the Struggle for a Rubber Monopoly, Encounter Books, New York City, BY, USA, 2002. ISBN-13: 978-1893554375
[8] Timeline, www.bouncing-balls.com (archived version accessed July 3, 2023)
[9] Hans-Georg Elias, Polymere: Von Monomeren und Makromolekülen zu Werkstoffen. Eine Einführung, Hüthig & Wepf Verlag, Zug—Heidelberg—Oxford, 1996.
[10] K. S. Reinartz, L. W. Ruetz, Effective Protection of Natural Rubber, Natuurrubber 1997, 9, 5–7.
The article has been published in German as:
- Die Chemie des Luftballons,
Klaus Roth,
Chem. unserer Zeit 2005, 39, 282–289.
https://doi.org/10.1002/ciuz.200590054
and was translated by Caroll Pohl-Ferry.
The Chemistry of Balloons (and Rubber) – Part 1
The balloon is certainly not the most important product based on rubber, but it may be the prettiest
The Chemistry of Balloons (and Rubber) – Part 3
How latex is collected, transported, and converted to natural rubber
The Chemistry of Balloons (and Rubber) – Part 4
How balloons are made and why rubber products can contain harmful nitrosamines
See similar articles by Klaus Roth published in ChemistryViews