Corroles are aromatic tetrapyrrole macrocycles. They are similar to porphyrin but have a contracted core with a direct pyrrole–pyrrole connection. Corroles are interesting in the contexts of, e.g., coordination chemistry, catalysis, or medicinal chemistry. Much of the research into corroles focuses on triarylcorroles, while β-octaalkylcorroles are less frequently studied today. In the latter, the meso-position can be functionalized, for example, via the formation of 10-oxocorroles. Such oxocorroles have generally been prepared using strong oxidants.
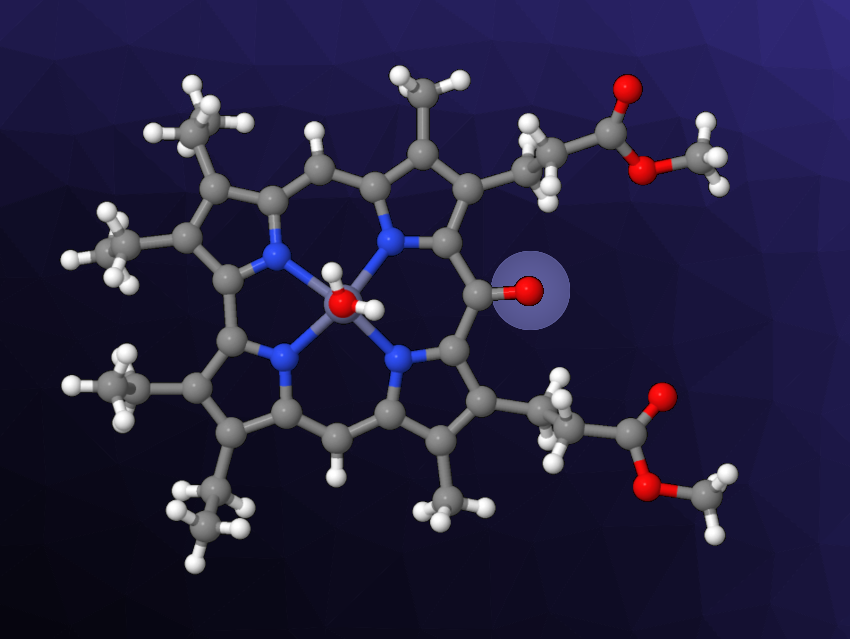
Sara Nardis, University of Rome Tor Vergata, Rome, Italy, and colleagues have found that oxocorroles can also obtained by zinc coordination to a β-octaalkylcorrole followed by air oxidation. The team reacted a substituted corrole with Zn ions in boiling methanol and found that two products were formed, namely, Zn complexes of the corresponding 5- and 10-oxocorroles (latter pictured). This pathway to oxocorroles does not require strong oxidants, instead relying on the oxygen in air.
The researchers also performed the reaction with octaethylcorrole as a substrate. As before, they obtained a mixture of the corresponding 5- and 10-oxocorroles. Due to the lower stability of the Zn complexes, however, they obtained oxocorrole free bases after the workup. Spectroscopic characterization confirmed that the prepared macrocycles have an antiaromatic character. According to the team, the simple synthetic protocol could be useful for more detailed studies of oxocorrole chemistry.
- 5- and 10-Oxocorroles from β-octaalkylcorroles,
Lorena Di Zazzo, Sara Nardis, Fabrizio Caroleo, Francesco Pizzoli, Frank R. Fronczek, Kevin M. Smith, Beatrice Berionni Berna, Roberto Paolesse,
Chem. Commun. 2023.
https://doi.org/10.1039/D3CC05204D