Humans often put on paper what is important to them—but paper is ephemeral. In Germany’s scientific libraries, for example, many of the 200 million books are disintegrating before our eyes. Nearly all the printed works produced between 1850 and 1970 are endangered, and some are already barely useable. Our archival materials, meaning all our official history, every page of which is unique and irreplaceable, are also in trouble.
This makes it worth looking into the chemistry of paper disintegration and the restoration of old paper.
1 The Beginnings of Paper Production
1.1 From China to Europe
In the year 105 CE, Chinese court official Tsai Lun reported a new discovery to his Emperor Ho Ti. He had used tree bark, bast fibers from mulberry trees, hemp, rags, and old fish nets to make a new, inexpensive material to write on: paper. The production process was extremely easy: the waste products were purified, shredded, pounded, boiled, and watered. The watery fiber pulp was skimmed off in a layer with a strainer, pressed, and dried [1]. The emperor was impressed by this product made from renewable resources (sustainable development) and used materials (recycling) that was fully biodegradable.
The first German paper mill was established near Nuremberg in 1390, and the paper there was produced in broadly the same manner as with the original Chinese method, with one exception: In Europe, paper was made exclusively from rags. Fabric rags, rope remnants, and nets were soaked for days in sodium or potassium carbonate and then mechanically broken down into individual fibers by a waterwheel-driven hammer mill. The paper was then formed with strainers.
The dried paper was very absorbent and completely unusable for writing because the ink would immediately bleed. Beginning in the 13th century, paper was sized (meaning treated with a filler or glaze) to reduce absorbency. Paper tissues and paper towels are examples of unsized paper.
The glue used for sizing was made by the paper manufacturers: Bones, hooves, horns, and tendons of slaughtered animals were boiled in a cauldron for a long period of time and the resulting liquid was filtered off. The paper was dipped into this bone glue or coated by brushing it on. The addition of alum [2] hardened (i.e., denatured) the bone glue, reducing the absorbency and swellability of the paper.
Alum is a double salt composed of potassium and aluminum sulfate (KAl(SO4)2•12 H2O). In paper manufacturing, it was later replaced by aluminum sulfate Al2(SO4)3•18 H2O, though the term “alum” was retained (papermaker’s alum). Bone glue consists mainly of gelatin, a protein rich in glutamic acid (side chain: –(CH2)2–COOH) and lysine (side chain: –(CH2)4–NH2). The Al3+ ions bind to the side chains, partially breaking up the three-dimensional network of hydrogen bonds in the native gelatin, denaturing the proteins. This hardens the gelatin gel.
1.2 The Hunt for Rags
After the invention of printing (see Infobox 1), the paper industry boomed, and rags became scarce. It is hard to imagine today, but from the end of the 15th until the middle of the 19th century—for nearly 400 years—there was a constant shortage of rags. Rag pickers were only allowed to work if they had an official license, and were forbidden to export rags in order to protect domestic industry (see Fig. 1). These draconian measures were necessary because the rag shortage was so great that in Prussia, scarecrows were taken from farmers’ fields.
 |
|
Figure 1. One illustration of the 400-year raw materials shortage in paper manufacture is this edict: In it, Friedrich II (Old Fritz) forbade the export of rags and all raw materials for glue production (vellum, skins, sheep’s hooves). According to this proclamation, rag pickers sworn in and licensed by the state “who are overcome by the desire to deliver their collected rags to anyone other than domestic paper makers or their appointed agents in contradiction of their oath, or to practice outside of the country will be punished by three months of forced labor (!!) in addition to the penalty for perjury.” |
Infobox 1. Gutenberg and Printing Ink.
(click here to expand)
Book printing was not made possible by a single stroke of genius, but rather several inventions. Multiple strikes of a letter stamp made of a hard metal onto a soft copper plate allowed Johannes Gutenberg (about 1397–1468) to cast many identical letters at the same time. In his press, a heavy metal plate ensured uniform pressure of the set type against the paper, a requirement for high-quality print.
 |
|
Figure B1. Johannes Gutenberg in a posthumously imagined portrait from the 16th century. No authentic images of Gutenberg survive. |
Gutenberg then tinkered for a long time with a quick-drying and viscous ink based on linseed oil that—unlike conventional aqueous inks— would not bleed through the paper. This allowed for double-sided printing. His formulation remained a secret, though it was possible to somewhat reconstruct its composition from calculations made in 1480 by a Florentine printing shop: First, a varnish was produced by heating the linseed oil, then soot, pitch, pyrite, cinnabar, resin, turpentine, gall nuts, iron salts, and shellac were mixed in.
The consequences of the restrictions were predictable. Rags were stolen, smuggled, and sold on black markets. The hunt for rags took on curious forms: In 1666, shrouds made of linen were forbidden in England because it was expected this would result in an additional 200,000 pounds of linen rags.
As late as 1800, the Westphalian Gazette reported about a calculation of paper millers: “They calculate that over ten years in just a single city where 3,000 people die in a year, 90,000 pounds of fine linen fabric, from which superb paper could be made, is irresponsibly fed to the worms in the soil.”
Most macabre was Mr. Stanwood’s business idea: As the owner of a papermill, he imported several shiploads of mummies from Egypt during the American Civil War and processed their linen wraps to make paper. This business idea backfired when the mummies also brought in cholera.
2 The Beginnings of Industrial Paper Production
2.1 Simplified Paper Production
For centuries, many clever people searched for a replacement for rags. Fame and fortune awaited those who succeeded. In the 18th century, Jacob Christian Schäffer, a scholar who has been almost completely forgotten today, published six volumes entitled “Experiments and Models for Making Paper Without any Rags or with only a Small Addition Thereof.”
Through years of arduous labor, he had investigated all possible plants to see if they were suitable for producing paper: from hop vines to stinging nettles, grapevines to sawdust, peat to red cabbage stalks, and pinecones to mugwort. In the end, this effort was all in vain—he found no replacement for rags [3].
Despite its high price, the demand for paper grew steadily. In 1800, Nicolas-Louis Robert invented the mechanical continuous production of paper with a revolving strainer, replacing manual manufacture. To size the continuous sheets, watchmaker Moritz Friedrich Illig had an ingenious idea in 1807. According to his “Instructions for a Reliable, Inexpensive Method of Sizing Paper in Bulk” the paper was sized by a novel method during the straining process instead of afterward [4].
Instead of gelatin for sizing, he used alkaline salts of resin acids (rosin soaps) that precipitate when alum is added to cellulose fibers. Up to about 3 % rosin soap was added to the fiber pulp and then precipitated out by the addition of an excess of alum. From today’s point of view, there is a nice bit of applied coordination chemistry at work here (see Infobox 2).
Infobox 2. Moritz Illig’s (1777–1845) Paper Sizing Method
(click here to expand)
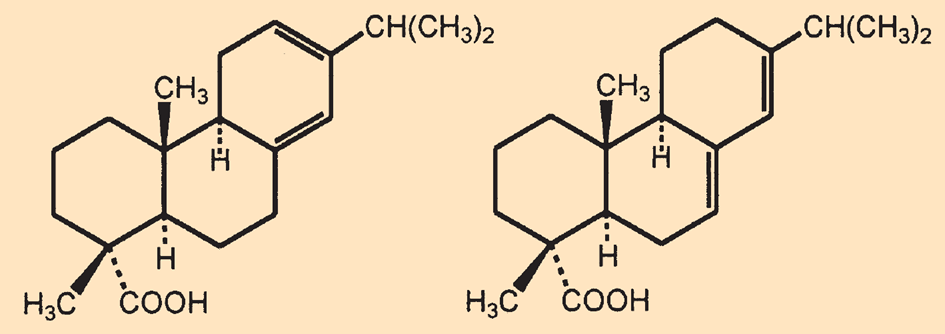
After the beginning of the 19th century, mechanically produced continuous paper was no longer sized with bone glue, but with rosin soap. The primary components of rosin are abietic and levopimaric acids (C20H30O2, see Fig. B2) and their esters. Paper makers boiled the rosin with soda (Na2CO3), partially saponifying the esters and forming the sodium salts of the resin acids (rosin soaps or resinates).
 |
|
Figure B2. Levopimaric (left) and abietic (right) acids. |
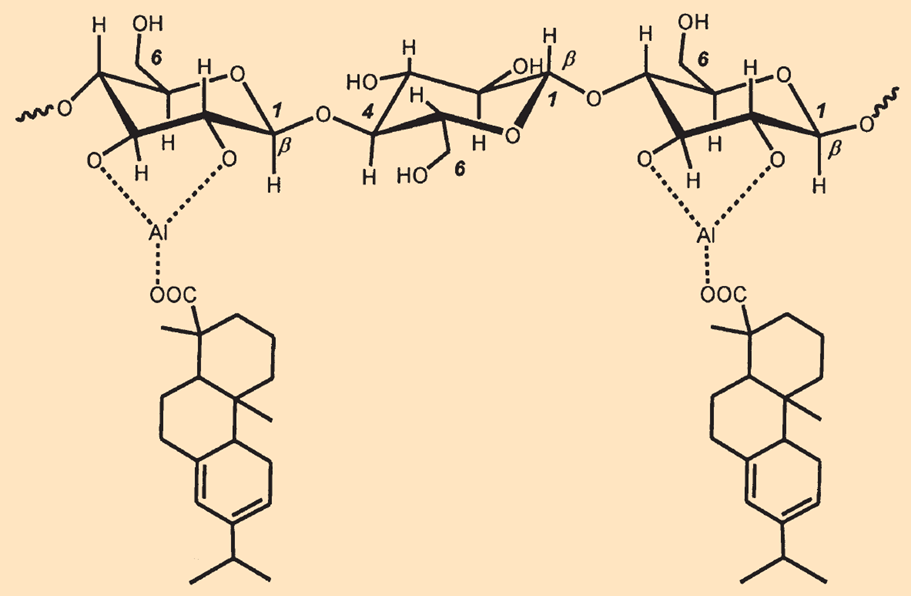
After addition of an excess of alum [KAl(SO4)2·12 H2O], the aluminum resinates precipitated out [B1]. Because aluminum cations can coordinatively bind to both the resin acid anions and the hydroxyl groups of the cellulose molecules simultaneously, the surface of the fibers is surrounded by the non-polar residues of the resin acids. This hydrophobic shell hinders the bleeding of aqueous inks (see Fig. B3).
 |
|
Figure B3. Aluminum cations Al3+ simultaneously bind to the resin acid anions and the hydroxyl groups in cellulose molecules. |
References
[B1] A.-C. Brandt, Mass Deacidification of Paper: A Comparative Study of Existing Processes, Bibliothèque National, Paris, 1992.
2.2 Wood Replaces Rags
Mechanical paper production and Illig’s sizing process simplified paper production, but the shortage of raw materials remained. The four-century search for a replacement for rags finally ended in 1840, when Saxon master weaver Friedrich Gottlob Keller first used very fine wood pulp to make paper. Because wood could be produced cheaply and in large quantities right outside his own front door, the paper industry experienced a breathtaking upswing that continues to the present day.
Unfortunately, this positive development came at a cost: Sizing based on rosin soaps/alum and the use of wood for paper production has led to the deplorable condition of our old written and printed records. This development is tragic, because from the point of view of the 19th century, these were truly revolutionary and beneficial inventions. They made paper into a mass product and allowed for the production of newspapers, books, textbooks, and writing paper that were available to everyone. We should, thus, not forget that many of the books and documents whose slow decomposition we bitterly bemoan today would not have been written or printed at all without the discoveries of Nicolas-Louis Robert, Moritz Illig, and Friedrich Keller.
References
[1] A. Pingel Keuth, Papierproduktion: Von Zellstoff zu Filtertüte, Schreibpapier, .., Chem. unserer Zeit 2005, 39, 402–409. https://doi.org/10.1002/ciuz.200500234
[2] K. Garlick, A Brief Review of the History of Sizing and Resizing Practices, The American Institute for Conservation, BPG, Washington, DC, USA, 1986. (accessed October 2, 2023)
[3] W. Sandermann, Papier: Eine spannende Kulturgeschichte, Springer Verlag, Heidelberg, Germany, 1992. https://doi.org/10.1007/978-3-662-09193-7
[4] G. Dessauer, Papierzerfall: Ursachen und Konsequenzen, Forum Bestandserhaltung. (archived version accessed October 2, 2023)
The article has been published in German as:
- Papierkonservierung: Chemie kontra Papierzerfall,
Klaus Roth,
Chem. unserer Zeit 2006, 40, 54–62.
https://doi.org/10.1002/ciuz.200600376
and was translated by Caroll Pohl-Ferry.
Chemistry Takes on Paper Conservation – Part 2
The chemical causes of paper disintegration
Chemistry Takes on Paper Conservation – Part 3
How deacidification can save old papers
See similar articles by Klaus Roth published on ChemistryViews.org



