Humans often put on paper what is important to them—but paper is ephemeral. This makes it worth looking into the chemistry of paper disintegration and the restoration of old paper.
In this part, we will consider the chemical causes of paper disintegration.
3 Causes of Paper Degradation
Like all organic materials, paper cannot escape its thermodynamic fate: It will eventually be oxidized in the presence of oxygen to give carbon dioxide and water. The question is not whether paper will age, but how fast.
There are many factors that accelerate this oxidation process: poor storage conditions such as high temperatures and humidity, light, air pollution, mold,  insects, and human stupidity. Human stupidity was and is the greatest foe of all cultural artifacts. The great library of Alexandria, in which the knowledge of the ancient world was stored for future generations, was destroyed by Caesar and then finally by Kalif Omar I in 640 C.E. Nothing was learned from this: Considering them pagan works of the devil, Louis the Pious incinerated all of the Germanic heroic tales collected by his father, Charlemagne, the Spanish burned almost all of the codices of the advanced Central American civilizations, in World War I, the German army destroyed the Library of Leuven in Belgium, Hitler burned books by Jewish and unpopular authors, and the Bosnian-Serbian army destroyed the library of Sarajevo in 1993. The examples are endless.
insects, and human stupidity. Human stupidity was and is the greatest foe of all cultural artifacts. The great library of Alexandria, in which the knowledge of the ancient world was stored for future generations, was destroyed by Caesar and then finally by Kalif Omar I in 640 C.E. Nothing was learned from this: Considering them pagan works of the devil, Louis the Pious incinerated all of the Germanic heroic tales collected by his father, Charlemagne, the Spanish burned almost all of the codices of the advanced Central American civilizations, in World War I, the German army destroyed the Library of Leuven in Belgium, Hitler burned books by Jewish and unpopular authors, and the Bosnian-Serbian army destroyed the library of Sarajevo in 1993. The examples are endless.
Even with proper storage, however, paper inevitably becomes brittle with age. Even a layperson can test it: If the corner of a page breaks off after being creased twice, the paper is designated as “vulnerable'”, if it breaks off after only slight bending, it is “unusable”. This folding test is easy to carry out, but only partly simulates the stress of normal use. It does at least allow for a qualitative evaluation of the mechanistic resilience of the paper being examined.
To this day, properly stored written records and prints from the late Middle Ages and Renaissance have lost almost none of their original beauty. However, things look different for paper produced after 1850. This paper ages dramatically and rapidly, becoming increasingly brittle. Let us compare paper production before and after 1850 from a chemical point of view [5].
3.1 The Chemistry of Paper Production Before and After 1850
Before 1850, the material basis of paper was exclusively fabric scraps made of linen or cotton. Alkaline maceration and mechanical pounding in paper mills resulted in long fibers of almost pure cellulose, which felted when strained and dried. In the elementary fibrils, the individual cellulose chains were held in a parallel arrangement by hydrogen bonds, forming some areas of highly ordered crystalline material. This increased the durability and chemical resistance of the paper because the inner cellulose strands were not directly accessible to aggressive external agents.
In contrast, for use in paper production, wood has to be ground up to an extreme extent (wood pulp), which very much shortened the lengths of the fibers from the outset. In addition, the cellulose from wood has a significantly smaller number of crystalline regions in comparison to linen or cotton. Most importantly, however, wood has a completely different chemical composition: beechwood, for example, contains 40 % hemicellulose and 20 % lignin in addition to its 40 % cellulose (see Infobox 3).
Infobox 3. The Structures of Cellulose, Hemicellulose, and Lignin
(click here to expand)
At about 1,011 t/y, cellulose (see Fig. B4) is quantitatively the most biosynthesized naturally occurring substance. It is made of several thousand glucose units (C6H12O6) linked in a chain. Cellulose is a poly(β-1,4-glucopyranosylglucopyranoside), meaning that the glucose units are present in the pyranose form (six-membered ring with an oxygen atom), and neighboring rings are linked through an oxygen bridge between the C-1 and C-4′ atoms. The β descriptor is used to indicate the equatorial position of the hydroxyl group in the 1 position.
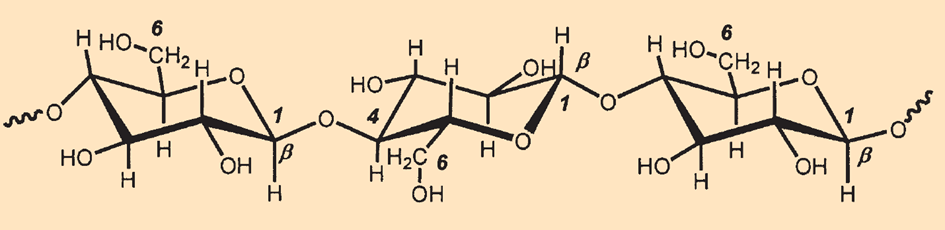 |
|
Figure B4. The structure of cellulose. |
This configurational specification is necessary because polyglucose with the α configuration (i..e, axial hydroxyl groups) is commonly known as starch and has completely different chemical properties.
Hemicellulose (see Fig. B5) is a heterogeneous polysaccharide in which other hexoses (C6H12O6) such as galactose and mannose, as well as pentoses (C5H10O5) such as xylose and arabinose, are linked together with glucose. In addition, this polysaccharide is branched by 1,2′ oxygen bridges, and the hydroxyl groups are partially acetylated and methylated.
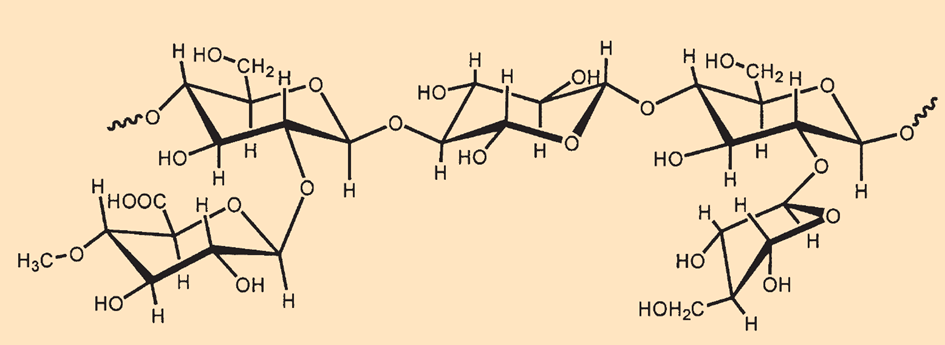 |
|
Figure B5. The structure of hemicellulose. |
Because some of the primary –CH2OH groups in the hexoses are oxidized to carboxyl groups (–COOH), hemicelluloses are soluble in alkaline solutions.
Lignin (see Fig. B6) is a polymeric phenylpropane derivative. Depending on the type of wood, the aromatic ring may have one or two methyl substituents in addition to the phenolic OH group. Because the phenolic hydroxyl groups can be deprotonated, lignin dissolves in alkaline solutions, allowing it to be separated from cellulose.
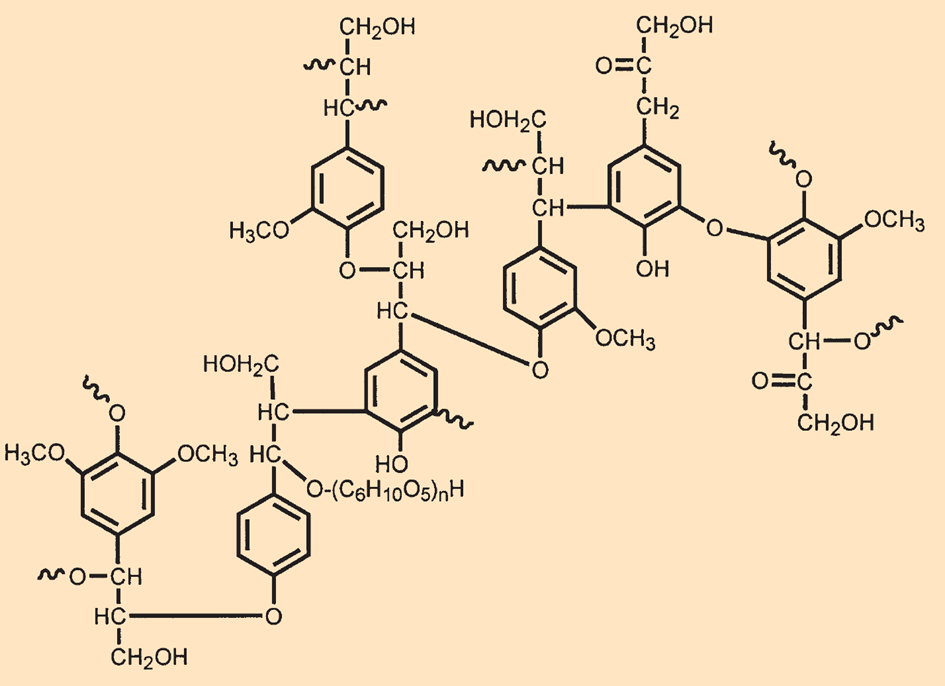 |
|
Figure B6. The structure of lignin. |
It is primarily lignin that reduces the quality of wood-based paper, because the elementary fibrils of the paper are surrounded by relatively nonpolar lignin and, therefore, cannot form any hydrogen bonds with each other.
It eventually became possible to separate lignin from wood through chemical processes, however, this “wood-free” paper never attained the same quality as that made from rags. “Wood-free” paper is still made from wood. In addition, the sizing process for this paper particularly favored degradation.
In the simplest sizing method, wood pulp was treated with strong lye. This deprotonates the phenolic groups (Ar-OH) in the lignin and the carboxyl groups (COOH) in the hemicellulose. Both polymers dissolve and can be separated from the cellulose (natron or soda pulp). Other processes (sulfite, sulfate, and organosolv processes) for obtaining the purest cellulose possible were developed later [5].
Before 1850, rags were processed by alkaline digestion with soda or potash to obtain the fibrous material, which resulted in alkaline paper. The trace amounts of alum used in sizing barely lowered the pH value because the bone glue acted as a large buffer reservoir.
After 1850, alkaline rosin soaps and a large excess of aluminum sulfate were added to the fiber pulp in the sizing process. With pH values between 4.5 and 5.5, the fiber pulp was acidic because, as the salt of the weakly basic aluminum hydroxide and strong sulfuric acid, the excess aluminum sulfate lowered the pH value.
3.2 Why Does Acidic Paper Degrade over Time?
Dry paper still contains about 5 % residual water, so the cellulose fibers in paper remain in contact with acidic water from the moment a sheet is produced. This cannot go well for long, because the glucose units in the cellulose chains are bound to each other through acetal bonds. Acetal groups are hydrolyzed in the presence of aqueous acid to form a hemiacetal and an alcohol (see Infobox 4).
Infobox 4. The Chemical Degradation of Cellulose
(click here to expand)
The C1-C4′ oxygen bridges between the glucose units in cellulose are each part of an acetal group R2C(OR1)(OR2). In the presence of acids, the neutral (acetal –OH) form is in equilibrium with the protonated (acetal–OH+) version (see. Fig. B7, left side, top to bottom).
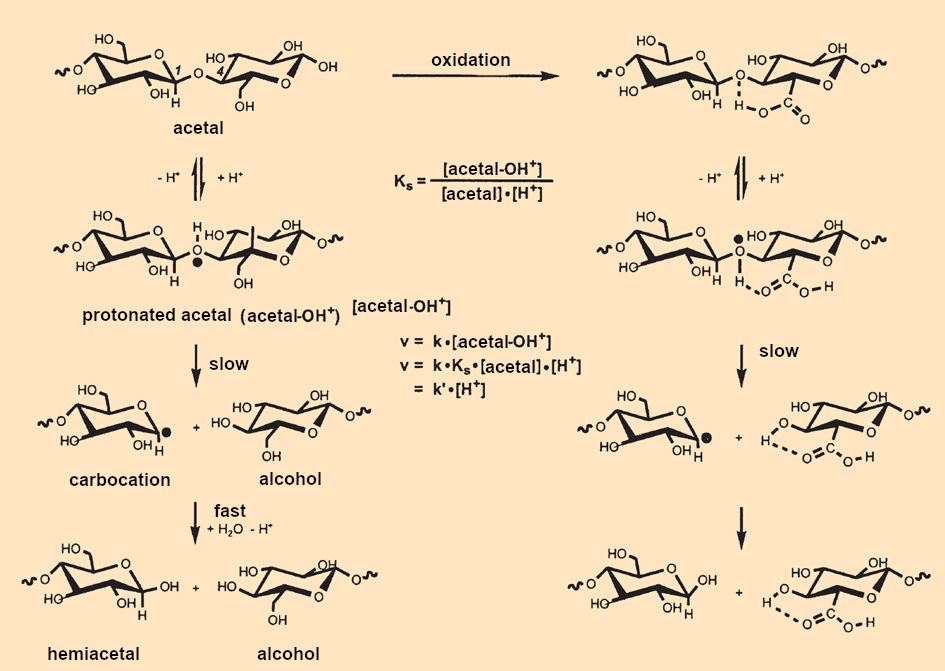 |
|
Figure B7. Chemical degradation of cellulose. |
In the slowest reaction step, the C1–O bond of the cation is broken [B2]. The subsequent reactions, namely attachment of a water molecule and splitting off of a proton, are very fast, resulting in one chain fragment that ends in a hemiacetal group R2C(OR1)(OH) and another that ends in an –OH group.
Because splitting the (acetal–OH+) cation is the rate-determining step, the rate of the reaction depends only on the concentration of this cation. The acetal group is a weak base, meaning the concentration of unprotonated acetal groups in cellulose is very high and remains essentially constant throughout the reaction.
Because the concentrations of the (acetal–OH+) cation and protons (pH value) are linked through the equilibrium constants, the rate of decomposition of cellulose is proportional to the proton concentration. The consequences are clear: a reduction of the pH value from 7 to 5 increases the rate of chain breakage by a factor of 100. It only takes a few breaks in each cellulose chain to noticeably reduce the tear strength of the paper.
The oxidative aging of paper initially causes the primary alcohol group (–CH2OH) to be oxidized by oxygen to form a carboxylic acid group (–COOH). This increases the proton concentration, accelerating decomposition. In addition, the protonated acetal cation (acetal–OH+) is energetically stabilized by a hydrogen bridge to the COOH group, increasing its concentration in the equilibrium relative to that in unoxidized cellulose. This causes Ks to increase, which also accelerates cellulose degradation.
Reference
[B2] D. P. N. Satchell, R. S. Satchell, Mechanisms of hydrolysis of thioacetals, Chem. Soc. Rev. 1990, 19, 55–81. https://doi.org/10.1039/CS9901900055
In the case of cellulose, this reaction leads to a break in the chain. Because the mechanical stability of paper depends on the felting of long fibers, breaking only one in a thousand of the bonds between the glucose units significantly reduces the paper’s tear strength. The experience of conservators has demonstrated that paper already begins to degrade at a pH value of 6 [6].
From a chemical point of view, paper produced after 1850 does not stand a chance against the ravages of time. Even worse, between 1850 and 1970, industrially produced paper was not only acidic from the moment it was produced, but it also became more acidic over time. Several factors were responsible for this.
The oxidation products of cellulose are acidic. For example, the primary alcohol groups in the 6-position of cellulose are oxidized to carboxylic acids [4]. The rate of these oxidation processes depends on the storage conditions (humidity, temperature, air quality). The climate in Manhattan, New York, USA, is clearly not good for books, as revealed by a comparison with identical volumes in libraries in California or Chicago [7]. Some volumes in the Bavarian State Library that were purchased from a library in New York after the Second World War are in significantly worse condition than corresponding examples in other German libraries [8].
The different storage temperatures and/or increased sulfur dioxide pollution in the air of New York could be responsible for this. In addition, aluminum sulfate undergoes a very complex hydrolysis process because the initially formed aluminum hydroxide is slowly dehydrated and polymerizes to insoluble products. The resulting sulfuric acid residue then further reduces the pH value over time (see Infobox 5).
Infobox 5. Hydrolysis of Aluminum Sulfate in Paper
(click here to expand)
In aqueous solutions, excess aluminum sulfate Al2(SO4)3 dissociates completely into its composite ions (see Fig. B8, Eq. 1). As the salt of a weak base (see Eq. 2) and a strong acid, aluminum sulfate reduces the pH value of paper to 4.5–5.5.
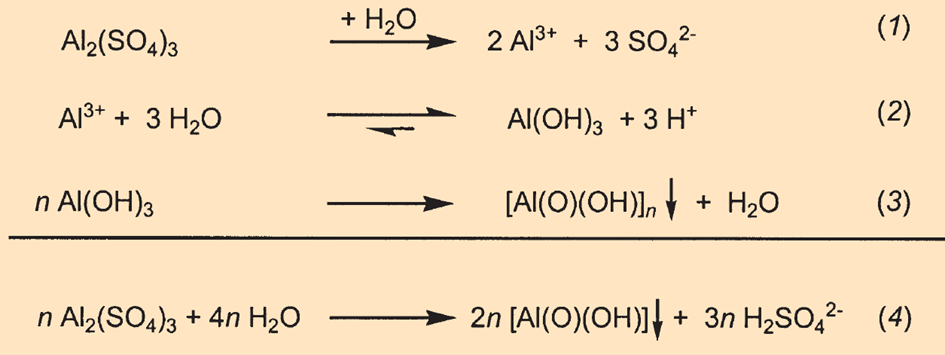 |
|
Figure B8. Hydrolysis of aluminum sulfate in paper. |
The hydrolysis processes involving Al3+ ions are even more complicated. Initially, Al3+ ions are coordinated to a varying number of hydroxyl ions or water molecules, depending on the pH value: Al(H2O)63+ (pH < 3), Al(OH)(H2O)52+ (pH < 3–7), Al(OH)2(H2O)42+ (pH < 4–8), Al(OH)3(H2O)3 (pH < 5–9) und Al(OH)4(H2O)2– (pH > 6) [B3].
Depending on the pH value, these ions release water and condense into dihydroxides or oligomeric hydroxides. In the end, this results in the formation of amorphous hydrous aluminum oxides of varying composition, which are insoluble in acids [B3] and accumulate on the fibers and in the spaces between them (see Eq. 3).
In short, polycondensation and the formation of insoluble hydrous aluminum oxides lead to the increasing acidification of paper (see Eq. 4).
Reference
[B3] Gmelins Handbuch der Anorganischen Chemie, Verlag Chemie (VCH), Weinheim, Germany, 1953, 35b, 98.
In summary, it has been established that the deterioration of paper is caused chemically by the acid-catalyzed splitting of cellulose. Paper produced between 1850 and 1970 was acidic from the outset due to the use of aluminum sulfate in the sizing process. Over time, aging of the insoluble hydrous aluminum oxides and the oxidative degradation of glucose lead to a further reduction in the pH value. In other words: Paper degradation is an autocatalytic process that goes faster and faster over time [9].
References
[5] S. T. Putnam et al., Papermaking additives, in Kirk-Othmer Encyclopedia of Chemical Technology: Noise Pollution to Perfumes, Wiley, 1981, 16, 768.
[6] H. Cheradame, S. Ipert, E. Rousset, Mass Deacidification of Paper and Books. I: Study of the Limitations of the Gas Phase Processes, Restaurator 2003, 24, 227. https://doi.org/10.1515/rest.2003.227
[7] Richard D. Smith, Deacidifying Library Collections: Myths and Realities, Restaurator 1987, 8, 69. https://doi.org/10.1515/rest.1987.8.2-3.69
[8] H. Bansa, Bibliotheksforum Bayern 1976, 4, 40.
[9] W. Dalrymple, A Paper Chase: Technology Helps Library Save Its Paper Collection, Libr. Congr. Inform. Bull. 1997, 56, 148.
The article has been published in German as:
- Papierkonservierung: Chemie kontra Papierzerfall,
Klaus Roth,
Chem. unserer Zeit 2006, 40, 54–62.
https://doi.org/10.1002/ciuz.200600376
and was translated by Caroll Pohl-Ferry.
Chemistry Takes on Paper Conservation – Part 1
The beginnings of paper production—from rags to wood
Chemistry Takes on Paper Conservation – Part 3
How deacidification can save old papers
See similar articles by Klaus Roth published on ChemistryViews.org




