Despite their danger, chlorates are still produced on an industrial scale and play an important, mostly unnoticed role in our day-to-day life. This merits a closer look.
4 The Other Side of Chlorates
When he discovered potassium chlorate in 1788, Claude Louis Berthollet not only observed its explosive power in mixtures with carbon, but also noted that its pure salts gave off oxygen upon heating. He thought this an elegant way to produce this gas on demand in the laboratory.
There was no way he could have known that his discovery would be used over 150 years later to save the lives of people suffering from acute oxygen deprivation. This side of chlorates is also largely unrecognized among chemists and deserves to be acknowledged here.
4.1 Oxygen Masks on Aircraft
Accidents involving sudden loss of oxygen happen in hostile environments such as submarines, diving bells, space stations, and mines. These days, people who often go to such places are trained in safety procedures and know what to do in an emergency. This is also true for passengers on commercial aircraft. Before takeoff (see Fig. 4) they are informed about what to do if there is a lack of oxygen due to a loss of cabin pressure. Most passengers do not pay attention and think this type of incident is unlikely. Unfortunately, this is not entirely true: the Aviation Medical Society of New Zealand documented 40–50 such incidents globally in the year 2000 [33].
 |
|
Figure 4. If cabin pressure suddenly drops. Left: Before takeoff, a flight attendant demonstrates how easy it is to use an oxygen mask. Right: In reality, a tangle of tubes drops from above (rubber jungle) with several oxygen masks for the passengers. (Image source: Matthew Bruch, US Air Force, public domain, Samuel Ashfield, Science Photo Library T160/0230). |
4.2 Imaginary Flight Incident
Let’s take an imaginary flight where this type of incident occurs. While still on the ground with an outside temperature of 15 °C and air pressure of 1013 hectopascals (hPa), the following safety instruction comes from the on-board speaker system: “In case of a loss of cabin pressure, oxygen masks will automatically drop from the ceiling. If this happens, pull the masks toward you, and place the opening firmly over your mouth and nose. Please assist any children traveling with you after putting on your own mask.”
After takeoff, the air pressure decreases with increasing altitude (see Tab. 1). At an altitude of 2500 m, the air pressure is just 735 hPa, and this pressure is maintained in the passenger cabin throughout the flight. At a cruising altitude of 11,000 m, the cabin is still at a comfortable 735 hPa, though the pressure outside of the aircraft is 220 hPa and the temperature is –57 °C.
|
Table 1. Physical and physiological effects of altitude [34,35]. |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
Now let us have the incident begin: A leak or other technical error causes the cabin pressure to fall. The oxygen masks automatically drop down on us from the hatch over our seats [36, 37] (see Fig. 4 right).
In the worst case, the pressure subsequently equalizes with the outside atmosphere and cabin pressure sinks to 220 hPa. This is a threat to the passengers because each breath only brings 1/5 of the oxygen needed into the lungs. Humans cannot endure this for long, and after 20–30 seconds of useful consciousness, they pass out (see Tab. 1). The passengers must, thus, quickly grab the rubber jungle dangling in front of them, untangle it, and put on their mask. If they pull this off, they can breathe in as much oxygen as they would in fresh air at sea level. This averts the danger of oxygen deprivation.
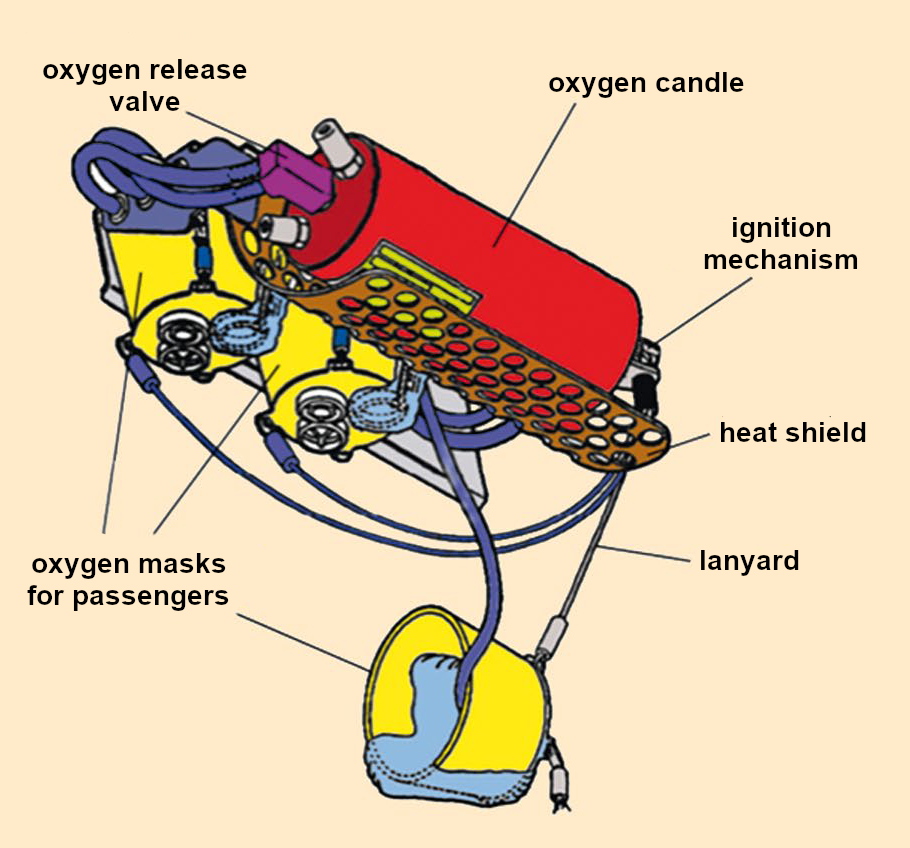
Once we are calmly breathing, we can look up to see where the oxygen in the tube comes from. A glimpse into the open hatch shows us that the oxygen source is a metal cylinder about the size of a thermos (25 cm long with a diameter of 7 cm) (see Fig. 5) [38,39]. This metal cylinder is known as a chemical oxygen generator or, more aptly, as an oxygen candle. It contains—and this takes our breath away—about a kilogram of sodium chlorate. This type of device is found above our heads in every row of seats. This could make even the most hardened inorganic chemists somewhat uneasy!
 |
|
Figure 5. Where does the life-saving oxygen come from? (Image source: The National Transportation Safety Board, wikimedia commons, public domain) |
A little chemical research will help us to relax: Pure sodium chlorate is thermodynamically stable, peaceful, and not at all explosive. It only becomes violent when exposed to reducing agents (carbon, sulfur, phosphorus, or organic materials).
Under an inert gas, pure sodium chlorate melts at 263 °C and remains unchanged in the melt over several days. Beware, however: Do not try to reproduce this! Mere traces of impurities can have a truly devastating effect.
During an incident-free flight, the oxygen candle does not present any danger as long as the sealed stainless-steel container perfectly protects the sodium chlorate from reducing agents.
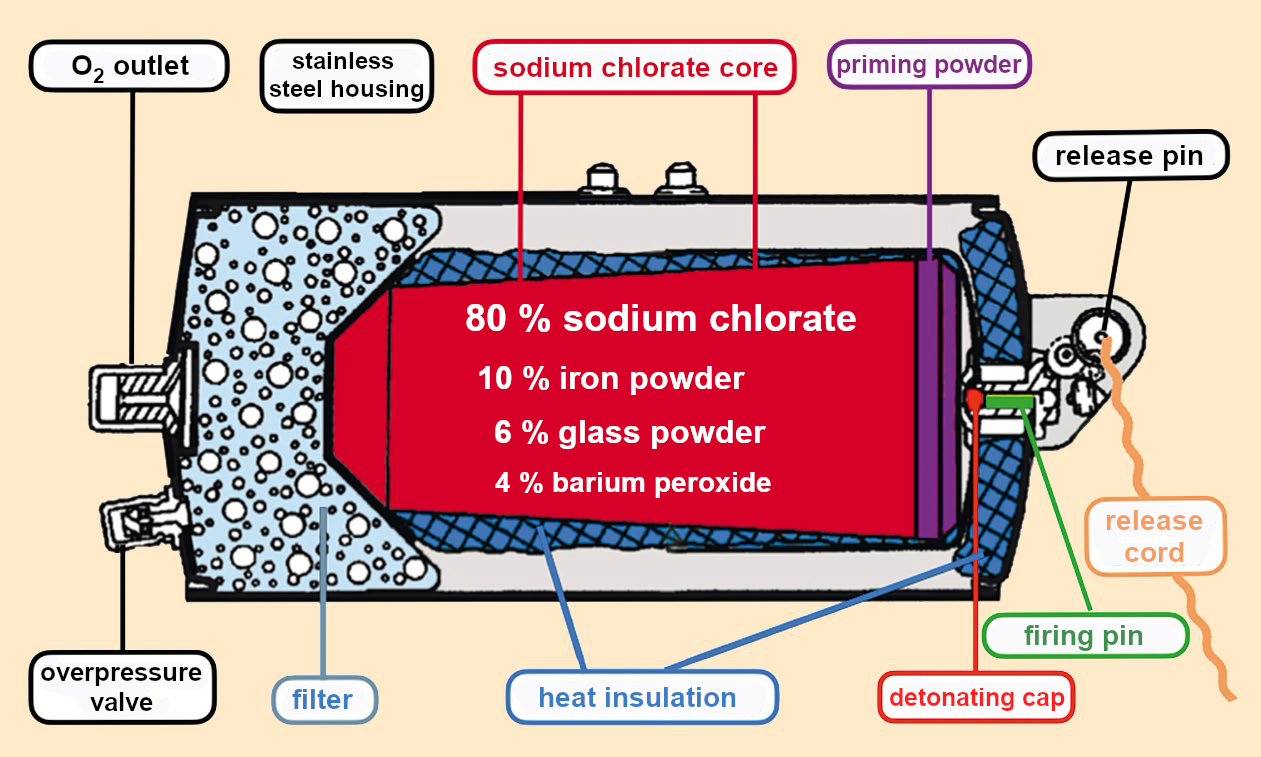
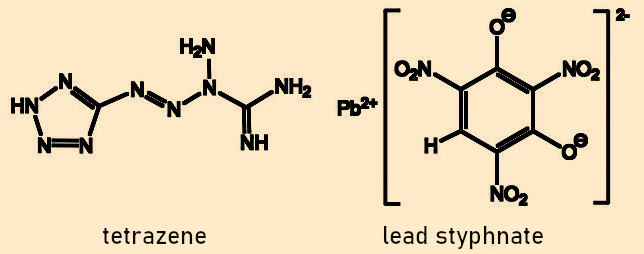
When we pulled on the oxygen masks, we unknowingly pulled out the release pin with a cord (see Fig. 6). This releases a taut spring, causing a firing pin to strike a detonator that contains a pressure-sensitive mixture of lead styphnate and tetrazene (see Fig. 7). The lead salt of styphnic acid acts as a primary explosive to ignite the explosive booster. Tetrazene is added to the lead styphnate because its pressure sensitivity greatly increases the reliability of the detonator.
 |
|
Figure 6. Chemical oxygen generator (Example Airbus A 321) (Image source: based on work by Jan Köhler [40]). |
 |
|
Figure 7. Primary explosive for oxygen generator. |
4.3 Chemical Oxygen Generator
When the detonating cap explodes, the heat of reaction is enough to cause the neighboring layer of the priming powder to react. This priming powder consists of a sodium chlorate/iron powder mixture with a high iron content. It undergoes a strongly exothermic reaction, warming to temperatures over 600 °C.
NaCIO3 + Fe → NaCI + FeO + O2 + heat (1)
This chemical heating [41] initiates the actual production of oxygen in the chlorate core. Complete oxidation to form iron(III) oxide (Fe2O3) does not occur. Instead, the reaction ends at FeO. The sodium chlorate melts and the following reactions take place [42]:
2 NaClO3 → 2 NaCl + 3 O2 ↑ (2)
4 NaClO3 → 3 NaClO4 + NaCl (3)
In a subsequent reaction, the sodium perchlorate decomposes, giving off oxygen:
3 NaClO4 → 3 NaCl + 6 O2 ↑ (4)
Although the multistep decomposition process that occurs in the molten sodium chlorate in equations (2) through (4) remains unclear, in the end, there is a seemingly simple overall reaction:
2 NaClO3 → 2 NaCl + 3 O2 ↑ + heat (5)
4.3.1 Catalyst
Reaction steps (2) through (4) are exothermic, but the heat released by the reaction is insufficient to keep the melt at the high temperature required for continued oxygen production. In consequence, the “sodium chlorate core” cools down and oxygen production stops. A catalyst would be helpful to allow the decomposition reactions to occur at lower temperatures.
Döbereiner first found manganese dioxide to function as a catalyst in 1832 [43,44]. Countless experiments demonstrated that many metals and metal oxides have a catalytic effect. However, though the decomposition temperature could be lowered, this was not enough. The material continued to cool down, stopping the reaction.
To maintain the mixture at the required high temperature, iron powder was added to the chlorate core. It is oxidized according to equation (1), producing enough heat by chemical means. By adding glass powder as a filler, the shape of the core can be stabilized and the burn rate reduced. Experimentation revealed the optimal mixture to deliver a steady stream of oxygen over a period of 15–20 minutes [45,46].
The high interior temperatures lead to strong heating of the outer shell to over 250 °C. This often results in a slight burning odor in the cabin.
4.3.2 Undesirable Side Reactions
Unfortunately, these extreme reaction conditions also lead to undesired side reactions, including the formation of elemental chlorine (6) [47].
4 NaClO3 → 2 Na2O + 5 O2↑ + 2 Cl2 ↑ (6)
Because chlorine is extremely toxic to humans, it must be removed. The perfect solution to this problem proved to be the addition of barium peroxide (BaO2) because this peroxide captures the chlorine before it leaves the hot melt.
BaO2 + Cl2 → BaCl2 + O2↑ (7)
Researchers got lucky in this case because barium peroxide also proved to be a catalyst for the decomposition of sodium chlorate. It thus plays two roles, catalyzing the formation of oxygen while also purifying it.
The modern chemical oxygen generator is based on many ideas from clever engineers and scientists. Today, modern oxygen candles are a reliable, safe, and low-maintenance tool for emergencies involving a lack of oxygen. They are not only used by extreme mountain climbers, firefighters, submarine and space station crews, and in mine and cave rescue operations, but also by anyone on a commercial flight and in some medical applications.
Over the course of this enthusiastic chemical contemplation, our airplane must have long since attained an altitude of 2500 m and the pilot has asked us to remove our oxygen masks. As a precaution, we will land at the nearest airport to find the cause of the loss of pressure. Whatever the cause may have been, we have safely landed our flight of imagination. Thanks to sodium chlorate!
Acknowledgment
The author thanks Dr. H. J. Schugk, Berlin, Germany, for his aeronautical advice.
References
[33] Aviation Medical Society of New Zealand, Rapid decompression in air transport aircraft, web.archive.org. (accessed August 24, 2022)
[34] Joachim Scheiderer, Die Atmosphäre in Angewandte Flugleistung – Eine Einführung in die operationelle Flugleistung vom Start bis zur Landung, Springer, Heidelberg, Germany, 2008. https://doi.org/10.1007/978-3-540-72724-8_7
[35] Mark Wolff, Cabin Decompression and Hypoxia, PIA Air Safety Publication (accessed August 24, 2022)
[36] desertsmurf, Oxygen Masks Dropping, YouTube 2015. (accessed August 24, 2022)
[37] Brad Salsbury, Oxygen mask drop on a NEW CRJ-900, YouTube 2016. (accessed August 24, 2022)
[38] Dieter Scholz, Sauerstoffanlage, Skript Hochschule für Angewandte Wissenschaften Hamburg (HAW Hamburg) (accessed August 24, 2022)
[39] J. Graf, Chlorate Oxygen Generator (Oxygen Candle), Review of the History of Candle Development, To Be Presented at Subs in Space, Houston, TX, USA, 2017. (accessed August 24, 2022)
[40] Jan Köhler, Aufarbeitung und Beschreibung ausgewählter Flugzeugkomponenten, Hochschule für Angewandte Wissenschaften Hamburg, Germany, 2006. (accessed August 24, 2022)
[41] William H. Schechter, R. R. Miller, Robert M. Bovard, C. B. Jackson, John R. Pappenheimer, Chlorate Candles as a Source of Oxygen, Ind. Eng. Chem. 1950, 42, 2348–2353. https://doi.org/10.1021/ie50491a045
[42] Meyer M. Markowitz, Daniel A. Boryta, Harvey Stewart Jr., The Differential Thermal Analysis of Perchlorates. VI. Transient Perchlorate Formation during the Pyrolysis of the Alkali Metal Chlorates, J. Phys. Chem. 1964, 68, 2282–2289. https://doi.org/10.1021/j100790a043
[43] J. W. Döbereiner, Bemerkungen über die Zersetzung des Oxychlorsauren Kalis, Ann. Pharm. 1832, 1, 236–237. https://doi.org/10.1002/jlac.18320010205
[44] F. E. Brown, J. Austin Burrows, H. M. McLaughlin, The decomposition of potassium chlorate. i. spontaneous decomposition temperatures of mixtures of potassium chlorate and manganese dioxide, J. Am. Chem. Soc. 1923, 45, 1343–1348. https://doi.org/10.1021/ja01659a001
[45] pppoxygen, Chemical Oxygen Generator – what happens inside the O2PAK – emergency oxygen, YouTube 2013. (accessed August 24, 2022)
[46] Aviyaytor, Airplane Oxygen Generator, YouTube 2012. (accessed August 24, 2022)
[47] Jian-Guo Liu, Long-Zhe Jin, Na Gao, Sheng-Nan Ou, Shu Wang, Wei-Xiang Wang, A review on chemical oxygen supply technology within confined spaces: Challenges, strategies, and opportunities toward chemical oxygen generators (COGs), Int. J. Min. Met. Mat. 2019, 16, 925–937. https://doi.org/10.1007/s12613-019-1809-6
The article has been published in German as:
- Als Richard Buckleys Hose explodierte,
Klaus Roth,
Chem. unserer Zeit 2022, 56, 102–109.
https://doi.org/10.1002/ciuz.202100062
and was translated by Caroll Pohl-Ferry.
Chlorates: Tragic Incidents and Life-Saving Applications – Part 1
A tale of exploding trousers, herbicides, fireworks, and airplane oxygen masks
Chlorates: Tragic Incidents and Life-Saving Applications – Part 2
Historic chlorate explosions and modern accidents
See similar articles by Klaus Roth published on ChemistryViews.org



An excellent article making even an ordinary person know how oxygen masks in airplanes work. Also shows how important chlorine compounds are in day to day life.
Thank you very much