Dental enamel is the hardest material in the human body. However, it is still possible for teeth to be damaged, for example, through cavities or consuming acidic foods. Therefore, modern dental hygiene is an important aspect of health care in general. In this part, we take a closer look at good dental hygiene and explain how toothpaste works.
4. Modern Home Dental Care
Most people make daily use of a toothbrush and toothpaste. If there is special interest in dental hygiene, then a mouthwash may also be used regularly. The latter ensures that all parts of the oral cavity will be affected, not just those conveniently reached by a toothbrush. For cleaning tight spaces between the teeth, dental floss or special tiny brush-like devices are recommended.
In addition to the traditional manual toothbrush, electric toothbrushes have gained increasing market share. In the foreground here stands a desire for a more thorough removal of bacterial biofilms—plaque—as a protection against tooth and gum inflammation. The choice of a toothbrush can influence the effectiveness of the toothpaste. With a round-headed brush, compared to the typical rectangular brush head, considerably less toothpaste will normally be involved, causing at least some of the many key ingredients (e.g., fluoride) to possibly be under-dosed [8].
Apart from the choice of a toothbrush, one’s cleansing technique (e.g., a modified Bass-rechnique (vibration technique: the bristle field is directed at an angle of about 45 degrees to the gum and moved back and forth) or Stillman-technique (the bristle field is pressed onto the gum at an angle of about 45 degrees to the tooth axis and the bristle field is rolled off towards the tooth) [5]) can also play a significant role, as does the duration of the brushing process. The frequency and extent of daily brushing may actually be less important than the brushing technique. An incorrect brushing method cannot be compensated for even with a lengthier brushing regimen.
As far as an optimal time of day for tooth brushing, no clear guidance is actually available. Brushing immediately after meals encourages removal of food residues, which does foster tooth hygiene. On the other hand, tooth enamel may have softened a bit after consumption of certain foodstuffs, especially those that are somewhat acidic. In this case, immediate brushing can cause small amounts of softened enamel to be removed from the tooth surface. Individuals already suffering from erosion damage are strongly advised to delay brushing at least an hour after eating or drinking, until tooth enamel will have been regenerated through remineralization from saliva [9].
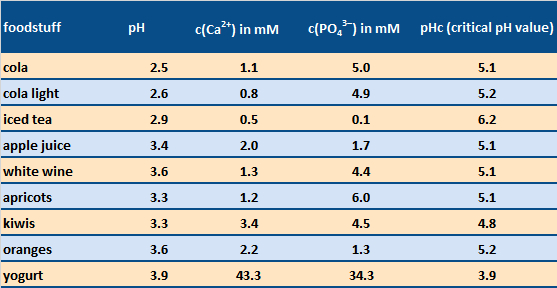
Daily toothcare mornings and evenings might be usefully supplemented by taking advantage of sugar-free chewing gum. The corresponding chewing action stimulates saliva flow, causing acidic pH conditions to be neutralized more rapidly. Note that a mouth pH value below ca. 5.5 inevitably leads to some dissolution of the solid substance of the teeth (see Tab. 5).
Ca5(PO4)3(OH) (solid) + 4 H+ (aq) ⇄ 5 Ca2+ (aq) + 3 HPO42– (aq) + H2O (l)
The common assertion that sugar-free beverages are best for the teeth can, in fact, be difficult to support, at least with respect to erosion by acid (see cola vs. cola light in Tab. 5), though an absence of sugar, of course, reduces the risk of cavities. Furthermore, the simultaneous presence of calcium or phosphate ions corresponding to the above solubility equation can lead to better tolerance relative to the acid present—as with acidic milk products like yogurt, or–in a limited manner—also with cola [10].
|
Table 5. Acidic foodstuffs which can have an erosive effect on the solid constituents of teeth [11]. Dissolution of tooth enamel can occur if one’s saliva pH after eating falls below the critical pH value pHc after consumption of the food. Foodstuffs with a high pHc value are, thus, particularly troublesome. |
 |
4.1 Toothpastes designed for specific target groups
Highly significant in private dental hygiene, apart from one’s toothbrush, is the nature of the toothpaste. A great variety of toothpastes is available commercially. Toothpastes differ primarily in the choice of contents, and are often designed for particular target groups: e.g., sensitivity toothpastes, whitening toothpastes, children’s toothpastes, etc. Alongside such specialty toothpastes, there is a vast array of “universal toothpastes”. (see also: What is a Good Toothpaste?)
Sensitivity Toothpastes
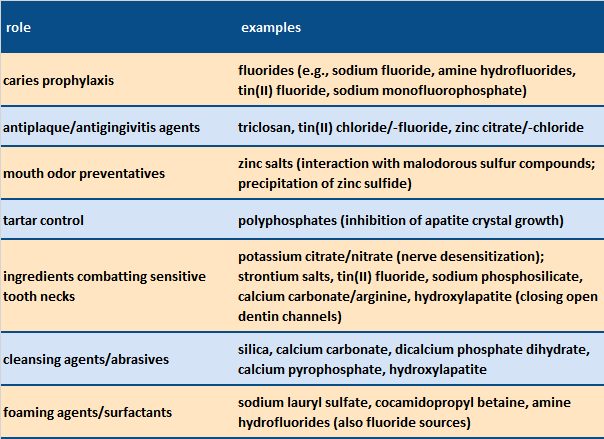
In addition to the typical components of a universal toothpaste (cleansing agents, thickeners, surfactants, and aromatic substances, including flavorings), toothpastes in the sensitivity category also contain desensitizers, such as potassium salts, or substances that close the dentin tubules, including hydroxylapatite or calcium carbonate (see Tab. 6). In general, sensitivity toothpastes also exhibit lower abrasion values (RDA values; see below) than universal toothpastes to protect exposed dentin.
|
Table 6. Major constituents of toothpastes [12]. |
 |
Children’s Toothpastes
Children’s toothpastes are distinguished by the fact that they also offer especially low abrasion characteristics. Moreover, they usually contain antimicrobial additives, like zinc salts or triclosan. Their fluoride content is generally limited to only 500 ppm, in contrast to 1450 ppm for adult toothpastes, in order to minimize potential toxicity risks from swallowing the toothpaste [13].
Modes of Action
The most common toothpaste additives for cavity (caries) prevention are fluoride-containing compounds. The plaque-removal effectiveness of a toothpaste depends primarily on cleansing agents in the formulation. Cleansers based on silica and calcium carbonate have achieved major market significance with respect to toothpastes in general. However, given the potential interaction of fluoride ions with calcium-containing cleansers (e.g., calcium carbonate, which leads to precipitation of CaF2), fluoride toothpastes typically contain only silica cleansers, unless a covalent fluoride compound is used, like sodium monofluorophosphate.
The choice of a cleanser also has a significant influence on both dentin abrasion and cleansing power, as expressed through the combination of the measured RDA (“radioactive dentin abrasion”) and PCR (“pellicle cleaning ratio”) values [14]. There are commercial toothpastes with low RDA values (as in the case of sensitivity and children’s toothpastes), modest RDA values (universal toothpastes), and high RDA values (whitening and smoker’s toothpastes).
Measurement of an RDA value
In order to establish an RDA value, tooth dentin is first irradiated in a neutron reactor. The result is radiochemical activation of phosphorus in the associated phosphate, consistent with the equation
3115P +10n → 3215P
This, in turn, results in nuclear decay with the emission of a β− particle (an electron), permitting phosphorus detection at very low levels.
3215P →3216S + 0–1β– (half-life t1/2 = 14.3 d)
There follows—with respect to the actual RDA analysis—the abrasion of dentin from the prepared teeth, using a slurry of toothpaste or cleansing agent in either water or artificial saliva (ca. 1,500 brush cycles). One then measures the radioactivity in the abraded substance, which will be proportional to the amount of displaced calcium phosphate. This is always followed by comparison with a reference abrasive (calcium pyrophosphate or silica), whose RDA value is defined as 100.
A high RDA value implies a high degree of dentin abrasion in the course of tooth brushing. RDA values above 250 are regarded as harmful with respect to teeth. Typical commercial toothpastes carry RDA values in the range 30—270 [15].
PCR Values
PCR values are established based on the discoloration of teeth (normally from cattle) by a staining agent—typically a tea/coffee mixture—applied to dentin and enamel. The color both before and after brushing is determined by light reflectivity measurements. The procedure itself provides a means for quantifying one specific aspect of the cleansing power of a toothpaste. Here again, a reference substance (typically the same one used in determining RDA values) is defined as having a nominal cleansing value of 100. Established PCR values for normal toothpastes range from about 25 to 140 [15].
RDA and PCR values are, of course, related, since both are based on the same effect (removal by brushing of stained tooth surface material). It thus becomes difficult to adjust the two independently.
An optimal cleansing agent produces a good compromise between effective cleansing (high PCR value) and minimal abrasion (low RDA). Recent developments show that toothpastes with equivalent cleansing power manage to accomplish their task with ever-lower levels of abrasion. This is one result of the use of modern silica cleansers (chemically: polysilicic acids SiO2 · n H2O) [14].
Silica Cleansers
As previously noted, an advantage of silica cleansers is that they are compatible with fluoride salts, in that the two do not react with each other (in contrast to the situation with calcium-containing cleansers). Moreover, through the availability of diverse precipitation techniques, many different types of polysilicic acids (silicas) are available, which can influence the abrasion and cleansing effects achieved with a toothpaste to a varying degree.
Apart from accomplishing the central task of plaque removal, a host of other substances can usefully be introduced into dental hygiene preparations, i.e., for protecting extra-sensitive teeth, or inhibiting mouth odor or bacterial growth (see Tab. 6).
References
[8] J. M. ten Cate, Contemporary perspective on the use of fluoride products in caries prevention, Br. Dent. J. 2013, 214, 161–167. https://doi.org/10.1038/sj.bdj.2013.162
[9] T. Attin, E. Hornecker, Tooth brushing and oral health: how frequently and when should tooth brushing be performed?, Oral Health Prev. Dent. 2005, 3, 135–140. https://doi.org/10.3290/j.ohpd.a10636
[10] A. Lussi, C. Ganss, Erosive Tooth Wear: From Diagnosis to Therapy, S. Karger, Basel, 2014. ISBN: 978-3-318-02552-1
[11] A. Lussi, T. S. Carvalho, The future of fluorides and other protective agents in erosion prevention, Caries Res. 2015, 49 (Suppl. 1), 18–29. https://doi.org/10.1159/000380886
[12] C. van Loveren, Toothpastes, S. Karger, Basel, 2013. ISBN: 978-3-318-02206-3
[13] Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products, Official Journal of the European Union, 2009.
[14] P. Wuelknitz, Cleaning power and abrasivity of European toothpastes, Adv. Dent. Res. 1997, 11, 576–579. https://doi.org/10.1177/08959374970110042701
[15] B. R. Schemehorn et al., Abrasion, polishing, and stain removal characteristics of various commercial dentifrices in vitro, J. Clin. Dent. 2011, 22, 11–18.
The article has been published in German as:
- Moderne Zahnpflege aus chemischer Sicht,
Matthias Epple, Joachim Enax,
Chem. unserer Zeit 2018.
https://doi.org/10.1002/ciuz.201800796
and was translated by W. E. Russey.
The Chemistry of Dental Care – Part 1
What teeth are made of and the causes of tooth decay
The Chemistry of Dental Care – Part 2
How to brush your teeth and how toothpaste works
The Chemistry of Dental Care – Part 3
Why fluoride is good for your teeth
See similar articles by Klaus Roth published in ChemistryViews Magazine