Dental enamel is the hardest material in the human body. However, it is still possible for teeth to be damaged, for example, through cavities or consuming acidic foods. Therefore, modern dental hygiene is an important aspect of health care in general. In this part, we take a closer look at why fluoride is good for your teeth.
5. Fluoride Compounds in Dental Hygiene
The use of fluoride-containing home tooth-care preparations is considered responsible for the considerable decrease in cases of cavities in recent decades [5]. In Europe, however, oral hygiene agents are legally classified as cosmetic resources, and are thus subject to the European Cosmetic Ordinance. This, in turn, specifies a maximum allowed concentration of fluoride. Thus, the highest permitted fluoride level in toothpaste is 0.15 % (calculated on the basis of F–).

The amount actually present is made clear on the packaging of a fluoride toothpaste intended for adults; a typical value is 1450 ppm fluoride (i.e., 0.145 %). Care is particularly called for with respect to children, since excessive fluoride levels can lead to fluorosis, a condition in which excess fluoride is incorporated into the teeth, leading to a yellowish discoloration.
Fluoride is the base corresponding to the moderately strong acid HF (hydrofluoric acid; pKa = 3.14). Under the conditions normally present in the oral cavity (pH = ca. 7) the equilibrium lies fully on the side of the free fluoride ion. Even under acidic conditions, which can be encountered in plaque (pH = ca. 5), fluoride is present predominantly as the free anion, not in the form of HF. The fluoride in domestic products for dental hygiene may be introduced in the form of various compounds. In Germany, the most common fluoride source at present is sodium fluoride.
Sodium Fluoride
The salt NaF is very water-soluble. In aqueous solution it dissociates into ions:
NaF (solid) → Na+ (aq) + F− (aq)
The nature of the counter-ion (sodium, potassium) here plays no significant role. Dissociation occurs immediately upon dissolution in water.
Tin Difluoride
SnF2 is a covalent material (i.e., not an ionic salt). The release of fluoride ions, in this case, requires hydrolysis, as expressed by the equation:
SnF2 (solid) + 2 OH− (aq) → Sn(OH)2 (aq) + 2 F− (aq)
This process occurs more rapidly at higher pH and higher temperature. Fluoride release here is clearly less rapid than with sodium fluoride, which dissociates into ions immediately upon dissolving.
Sodium Monofluorophosphate
Na2PO3F also dissociates immediately upon dissolving in water, according to the equation:
Na2PO3F (aq) → 2 Na+ (aq) + PO3F2− (aq)
Fluoride itself is then released by hydrolysis of fluorophosphate to phosphate:
PO3F2− (aq) + OH− (aq) → HPO42− + F− (aq)
Fluoride becomes available only if hydrolysis occurs sufficiently rapidly during the cleansing process (1–2 min). Alternatively, hydrolysis might take place subsequently, with fluorophosphate adsorbed onto the teeth. Hydrolysis can proceed both chemically and enzymatically [16].
Sodium monofluorophosphate is often used in combination with calcium-containing cleansing agents, since the precipitation of calcium fluoride occurs here to a much smaller extent than with sodium fluoride or amine hydrofluorides.
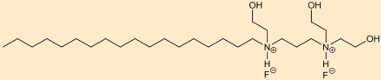
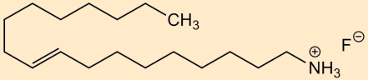
Amine Hydrofluorides
These are salts comprised of a cationic surfactant (ammonium salt) and the fluoride ion. Typical examples are:
- olaflur
- dectaflur
Dissolution in water leads to immediate and complete dissociation, corresponding to:
R+F− (aq) → R+ (aq) + F− (aq)
This dissociation is independent of the nature of the ions, i.e., the same occurs with chloride instead of fluoride as the counterion.
Effect of Fluorine
Fluoride has a prophylactic effect against caries. The cationic surfactant plays a bacteriocidic role (as is also true with cetylpyridinium chloride). Here, the bacteriocidal effect is not a function of the fluoride, as can be shown by the use of the corresponding amine hydrochloride [17].
It is also important to note that fluoride ions can be deactivated by calcium-containing cleansers, i.e., calcium carbonate:
2 F− (aq) + CaCO3 (solid) → CaF2 (solid) ↓ + CO32− (aq)
or calcium hydrogen phosphate:
2 F− (aq) + CaHPO4 (solid) → CaF2 (solid) ↓ + HPO42− (aq) (perhaps also giving fluorapatite, Ca5(PO4)3F)
5.1 Solution and Precipitation Equilibria in the Oral Cavity
The mode of action of fluoride can be readily understood from a chemical standpoint in terms of the solid phases present and their solubility products. The source of the fluoride is irrelevant, incidentally; what is instead always important is fluoride ions in solution.
In what follows, we provide various calculations related to both dissolved and solid phases in the presence of fluoride in saliva. It is important to us in this context that these calculations—without complex numeric solution procedures—suffice for taking into account subsequent precipitation, protolysis, and complex-formation equilibria. They will, thus, rely on a few simplifying assumptions, but for this reason remain readily intelligible.
We assume in what follows that all ions are present in pure water. The following numerical values are thus employed in the calculations:
- hydroxylapatite Ca5(PO4)3OH: S = 1 ∙ 10–58.8 M9 [1]
- fluorapatite Ca5(PO4)3F: S = 9 ∙ 10–61 M9 [18]
- phosphoric acid H3PO4: pKa values 2.16, 7.21, and 12.32 [19]
- calcium concentration in saliva: 1.43 mM [6]
- phosphate concentration in saliva: 5.10 mM [6]
- pH in saliva: 6.0–7.5 [5]; assumed in what follows to be 7.0
- fluoride concentration in saliva after brushing with toothpaste containing 1500 ppm of fluoride, diluted ca. 1:10: 150 ppm = 7.9 mM [6]
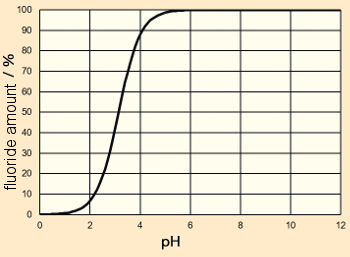
The fluoride ion is the base corresponding to the moderately strong acid HF (hydrofluoric acid, pKa = 3.14). It, therefore, follows that, depending upon the pH, a protonation may occur:
F– (aq) + H3O+ (aq) ⇄ HF (aq) + H2O (l)
If one calculates the extent of protonation as a function of pH, it turns out that, at pH 7, hydrofluoric acid is completely deprotonated (see Fig. 8). In all the calculations that follow we can, therefore, assume that the fluoride ion concentration present is equal to the total fluoride concentration, i.e., c(HF) ≈ 0.
x(F–) = Ka/(Ka + c(H3O+))
 |
|
Figure 8. Protonation level of fluoride as a function of pH. |
Let us now consider the source of fluoride in toothpaste: Release of fluoride from fluoridation agents is expected to occur in the following sequence:
sodium fluoride = amine hydrofluorides > tin(II) fluoride ≈ sodium monofluorophosphate
Consider now the not readily soluble phases that might be precipitated in the mouth:
Calcium fluoride is a slightly soluble salt (S = 3.9 ∙ 10–11 M3). It dissolves upon addition of acid, because the fluoride ion is removed from the equilibrium through protonation:
CaF2 (solid) → Ca2+ (aq) + 2F– (aq)
F– (aq) + H3O+ (aq) ⇄ HF (aq) + H2O (aq)
Combining the two equilibria makes it possible to calculate the equilibrium concentration of calcium as a function of pH:
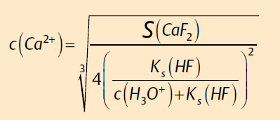
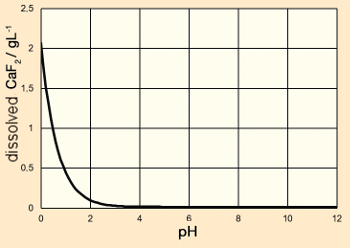
The dissolved quantity of calcium fluoride follows then stoichiometrically as a function of pH (see Fig. 9):
w(CaF2) = c(Ca2+) ∙ M(CaF2)
It is apparent that, under neutral pH conditions, CaF2 is nearly insoluble in water, including in the absence of additional fluoride (amount dissolved at pH 7: 16.7 mg L–1).
 |
|
Figure 9. Dissolved quantity of calcium fluoride in water as a function of pH. |
Fluorapatite is the second insoluble solid phase in this system. In a fluoride-containing environment, hydroxylapatite transforms itself completely into fluorapatite:
Ca5(PO4)3OH (solid) + F– (aq) → Ca5(PO4)3F (solid) + OH– (aq)
The greater thermodynamic stability reveals itself, among other ways, through the fact that fossil tooth and bone samples are converted almost completely into fluorapatite. Depending upon the environment, this occurs within a few 100 to 1,000 years. Substitution of fluoride for hydroxide can also occur to some extent, leading to the mineral francolite, Ca5(PO4)3(OH,F).
In what follows, we assess what solid phases should be present in contact with saliva. Critical here for all calculations in which phosphate plays a role is the fact that in solubility products, it is the concentration of orthophosphate, PO43–, that is involved. Below pH 12, this represents only a fraction of the overall phosphate concentration. It would, thus, be entirely wrong to introduce an overall phosphate concentration into the equation. Since phosphoric acid is a tribasic acid, three different protolysis equilibria must be taken into account, which is difficult from a mathematical-analytical point of view.
With a few assumptions, at pH 7 the orthophosphate ion concentration can easily be estimated. Thus:
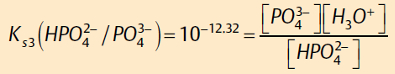
Rearranging gives:
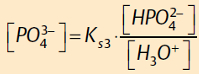
Therefore, at pH = 7:
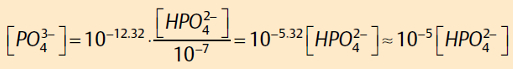
The phosphate concentration at pH 7 is roughly 10–5 times the hydrogen phosphate concentration. This is the value we will assume in further calculations.
At pH = pKs2 = 7.21, it is true that:
[H2PO4–] = [HPO42–]
Since the amount of phosphoric acid, H3PO4, at this pH, as well as the amount of orthophosphate can be ignored, it is true that:
[H2PO4–] + [HPO42–] = [total phosphate]
And correspondingly at pH 7, i.e., near to pKa2 = 7.21, it is true that:
[HPO42–] ≈ [total phosphate] /2 = 2.55 ∙ 10–3 M
(Total phosphate in the mouth: 5.1∙10–3 M; see above.)
Therefore:
[PO43–] = 2.55 ∙ 10–8 M at pH = 7.
Further calculations will be based on this value.
Let us next consider the solubility equilibrium for hydroxylapatite, Ca5(PO4)3OH; that is to say, the mineral substance in teeth. For the ion product, IP, the following applies:
IP = [Ca2+]5 [PO43–]3 [OH–] = (1.43 ∙ 10–3)5 (2.55 ∙ 10–8)3 10–7 M9 = 9.9 ∙ 10–45 M9
Supersaturation, as opposed to precipitation, can be calculated using:
![]()
At pH 7, saliva is therefore highly supersaturated with respect to hydroxylapatite. This is to be expected, since teeth do not dissolve under neutral conditions. However, it illustrates the possibility for remineralization of tooth enamel after attack by acid.
Consider now the solubility equilibrium S for fluoroapatite, Ca5(PO4)3F:
S = 9 ∙ 10–61 M9 [Ca2+]5 [PO43–]3 [F] = (1.43 ∙ 10–3)5 (2.55 ∙ 10–8)3 [F] M8 = 9.9 ∙ 10–38 [F] M8
Through rearrangement, we arrive at an equilibrium concentration in saliva of
[F–] = 6.5 ∙ 10–24 M
This means that saliva, even at the lowest fluoride concentrations, is supersaturated with respect to fluoroapatite. For saliva containing 150 ppm fluoride after brushing, it remains true that:
IP = [Ca2+]5 [PO43–]3 [F] = (1.43 ∙ 10–3)5 (2.55 ∙ 10–8)3 ∙ 7.9 ∙ 10–3 M9 = 7.8 ∙ 10–40 M9
with a supersaturation of
![]()
In the presence of fluoride, saliva is thus very highly supersaturated relative to the precipitation of fluoroapatite.
Finally, we consider the solution equilibrium of calcium fluoride, CaF2, in the presence of fluoride-containing saliva. For saliva containing 150 ppm fluoride after brushing, there applies:
IP = [Ca2+]5 [F] = 1.43 ∙ 10–3 ∙ (7.9 ∙10–13)2 M3 = 8.9 ∙ 10–8 M3
with a supersaturation of
![]()
After brushing with fluoride-containing toothpaste, it is thus possible that there will be precipitation of CaF2. But the supersaturation of fluoroapatite is much greater.
5.2. The Protective Effect of Fluoride Against Caries
Various mechanisms of action have been discussed with respect to the anticavity (anticaries) effect of fluoride (see Tab. 7) [5]. The central point in this context is the accelerated crystallization (nucleation) of calcium phosphate during remineralization. However, the incorporation of fluoride into the enamel during routine daily tooth care is extremely limited (fluoride content of 500–1,000 ppm in the outer layer of solid tooth enamel; a stoichiometric fluoroapatite would have 36,400 ppm = 3.64 %) [20, 21]. There is also no detectable evidence for an acid-insoluble protective layer of calcium fluoride or fluoroapatite [21]. As a result, the activity of fluoride is apparently due to the effects of dental hygiene itself.
|
Table 7. Postulated mechanism of action for fluorides in home dental care, with comments from a chemical point of view. |
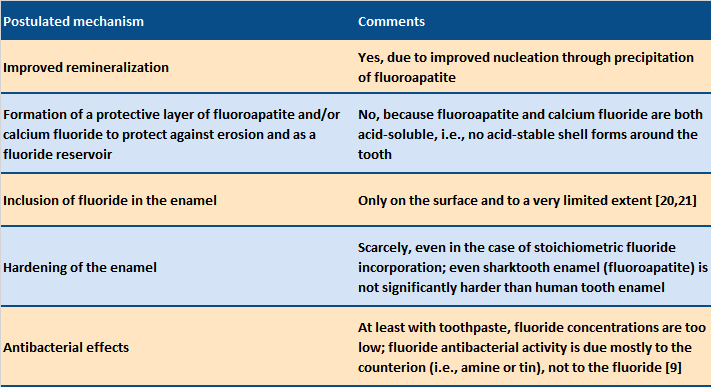 |
The discovery that the fluoride concentration is very low in the outermost enamel layer of the teeth, and that no crystalline calcium fluoride is detectable, indicates substoichiometric inclusion of fluoride in the hydroxyapatite lattice. Fluorapatite and hydroxylapatite are hardly distinguishable crystallographically, especially in the presence of extraneous ions [1]. One can conclude that, due to its higher degree of supersaturation, fluoroapatite crystallizes first, and acting as a seed, accelerates the further deposition of hydroxylapatite, without the incorporation of large amounts of fluoride.
Fluoride thus functions as a “catalyst” for the natural remineralization process from the saliva. Furthermore, the active agent fluoride requires saliva (i.e., the presence of calcium and phosphate ions) to be effective.
It is interesting to compare human teeth with shark teeth. The enamel of the shark tooth (enameloid) consists of almost stoichiometric amounts of fluorapatite, Ca5(PO3)3F [22]. Studies have shown that shark teeth, despite their high fluoride content, are subject to attack by acid [23]. Shark teeth are just as hard as human teeth [22], so that one cannot assume hardening of the enamel through an integration of fluoride after use of a fluoride-containing oral-care product. The antibacterial effect of fluorides is due mainly to the counterion (with amine hydrofluorides, the cationic amine, with tin fluoride the Sn(II) [9]), and not to fluoride itself. The fluoride concentration is too low to produce an effective antibacterial activity. Table 7 summarizes these findings.
6. Biomimetic Concepts in Dental Care
Alongside established concepts, modern dental care also recognizes novel biomimetic approaches [24]. Especially exciting are the search for insights inspired by nature (biomimetics), and optimization and the further development of existing concepts in dental care.
Biomimetic approaches are often based on calcium phosphates, such as hydroxylapatite, amorphic calcium phosphates, and tricalcium phosphates [12]. Due to its chemical similarity to the natural solid substance in teeth, hydroxylapatite is especially interesting as a biomimetic material in dental care preparations [25]. In contrast to fluorides (max. 0.15 % F–, see above), the concentration of hydroxylapatite in oral health preparations is not restricted by the European Cosmetic Ordinance, so that larger amounts of this active substance can be incorporated. This is of special interest with respect to children’s dental care.
There are numerous publications related to biomimetic dental care that involve hydroxylapatite, which among other things describe a reduction of the initial formation of microbial plaque on tooth enamel by hydroxylapatite particles [26]. Although biomimetic approaches are in general less well covered in the literature than studies with established substances (e.g., fluorides), there has been an increasing number of significant clinical studies demonstrating the effectiveness of these concepts [27]. From a chemical point of view, the inclusion of hydroxylapatite in dental care products is certainly advantageous, in the interest of facilitating the remineralization of tooth enamel (increasing the ion product of apatite).
7. Expanded Dental Care
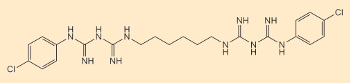
If normal household dental care is not sufficient (e.g., after orthopedic surgery on the jaw), additional preparations may be called for. The gold standard for control of biofilm is chlorhexidine (CHX, see Fig. 10) [5].
 |
|
Figure 10. Structure of chlorhexidine (CHX). |
In a professional setting, CHX is used either in the form of a mouth rinse, or as a varnish. CHX functions as an antibacterial agent by destroying bacterial cell membranes. Due to its special molecular structure, containing polar segments, CHX interacts extremely well with mucous membranes in the oral cavity, and thus offers a very high antibacterial efficiency. Among the undesired effects of CHX treatment is a possible discoloration of the teeth.
For patients at high risk of developing caries, high-dose fluoride preparations are often recommended (> 1500 ppm; i.e., not cosmetic agents, as defined by the European Cosmetic Ordinance). These may be used at home, or especially in a dentist’s office (fluoride varnish). In such cases, there have actually been reports of in vitro calcium fluoride precipitation on tooth surfaces [5].
8. Studies on the Efficacy of Dental Care
It is fundamentally very difficult to develop rigorous scientific proof for mechanisms of action associated with dental care. This is attributable to the nature of the problem: The environment of a tooth in one’s mouth is a very complex one, and is especially difficult to characterize. Apart from inorganic-chemical parameters like pH and ion concentrations, numerous organic and biological substances also play critical roles.
The oral bacterial flora, which are sources of dental plaque, differ from person to person, and depend—among other things—on dietary habits, dental care, and the place of residence (drinking water). Clinical tests are usually based on large numbers of test subjects in order to achieve adequate statistical significance. Fundamental insight into the underlying chemical, physical, and biological processes is usually available only to a very limited extent, since these processes unfold on the micrometer or nanometer scale. Studies directly in a patient, as with electron microscopy or elemental analysis, are quite impossible—except after the extraction, for example, of wisdom teeth. Thus, one often must make do with systems as near to reality as possible, perhaps involving extracted and polished teeth from cattle, which are then handled in the laboratory under conditions as near to physiological as possible (i.e., with artificial saliva).
One interesting possibility is the temporary (i.e., a few hours) and occasional introduction of simulated tooth surfaces into the mouths of human test subjects, as a way of exposing them there to a natural oral environment, and then, following removal, to analyze them with sensitive and sophisticated techniques—so-called in situ investigations [26]. A true simulation of chemical-biological conditions in the oral cavity is unfortunately only possible to a limited extent, given the extreme complexity, so that the resulting predictions are of limited value.
9. Outlook
Given the improvements in oral hygiene generally and the increased deployment of fluorides, the overall prevalence of tooth decay has decreased significantly. Nevertheless, tooth erosion damage has been increasing as a result of modern dietary habits (more frequent consumption of acidic beverages and foods). Gum disease is also widespread, with a frequency and severity that increases with age [7]. Modern prophylaxis is, therefore, an interplay between diet (limited consumption of sugar and acidic foods), domestic oral hygiene (thorough removal of plaque, active agents), and professional prophylaxis in the dentist’s office.
Future developments can be anticipated especially in the area of acid erosion. Here, both prevention and enamel regeneration play important roles. Given an increasingly aging population, dental care in the face of limited amounts of saliva becomes important (senile dental decay). For chemists, the oral hygiene field offers exciting interdisciplinary possibilities, encompassing many aspects of solid-state chemistry, solution chemistry, analysis, biochemistry, and microbiology.
References
[16] P. W. Heidbüchel, Fluor determination in toothpaste-extracts with fluoride-selectrode based on the kinetics of hydrolysis of sodiummonofluorophosphate, Pharm. Acta Helv. 1991, 66, 290–297.
[17] S. Shani et al., Relation between surface activity and antibacterial activity of amine-fluorides, Int. J. Pharm. 1996, 131, 33–39. https://doi.org/10.1016/0378-5173(95)04299-7
[18] K. O. A. Chin, G. H. Nancollas, Dissolution of fluorapatite. A constant-composition kinetics study, Langmuir 1991, 7, 2175–2179. https://doi.org/10.1021/la00058a034
[19] W. Buckel, Renaissance der Supraleitung, Phys. Unserer Zeit 1991, 22, 217–222. https://doi.org/10.1002/piuz.19910220509
[20] J. A. Weatherell at al., Changes in the fluoride concentration of the labial enamel surface with age, Caries Res. 1972, 6, 312–324. https://doi.org/10.1159/000259810
[21] F. Mueller et al., Elemental depth profiling of fluoridated hydroxyapatite: saving your dentition by the skin of your teeth?, Langmuir 2010, 26, 18750–18759. https://doi.org/10.1021/la102325e
[22] J. Enax et al., Structure, composition, and mechanical properties of shark teeth, J. Struct. Biol. 2012, 178, 290–299. https://doi.org/10.1016/j.jsb.2012.03.012
[23] B. Ögaard et al., Microradiographic study of demineralization of shark enamel in a human caries model, Scand. J. Dent. Res. 1988, 96, 209–211.
[24] M. Hannig, C. Hannig, Nanomaterials in preventive dentistry, Nat. Nano. 2010, 5, 565–569. https://doi.org/10.1038/nnano.2010.83
[25] J. Enax, M. Epple, Synthetic hydroxyapatite as a biomimetic oral care agent, Oral Health Prev. Dent. 2018, 16, 7–19. https://doi.org/10.3290/j.ohpd.a39690
[26] C. Hannig et al., Influence of a mouthwash containing hydroxyapatite microclusters on bacterial adherence in situ, Clin. Oral Investig. 2013, 17, 805–814. https://doi.org/10.1007/s00784-012-0781-6
[27] I. Harks et al., Impact of the daily use of a microcrystal hydroxyapatite dentifrice on de novo plaque formation and clinical/microbiological parameters of periodontal health, A randomized trial, PLoS One, 2016, 11, e0160142. https://doi.org/10.1371/journal.pone.0160142
The Authors

Matthias Epple is Professor of Inorganic Chemistry at the University of Duisburg-Essen, Germany. Among other things, he works with biological crystallization phenomena and the synthesis of nanoparticles.

Joachim Enax obtained his doctorate in 2014 in inorganic chemistry, on the characterization of shark teeth and the development of biomimetic dental composites. Since 2015 he has served as Senior Scientist for Oral Care at the firm Dr. Kurt Wolff GmbH & Co. KG in Bielefeld, Germany.
The article has been published in German as:
- Moderne Zahnpflege aus chemischer Sicht,
Matthias Epple, Joachim Enax,
Chem. unserer Zeit 2018.
https://doi.org/10.1002/ciuz.201800796
and was translated by W. E. Russey.
The Chemistry of Dental Care – Part 1
What teeth are made of and the causes of tooth decay
The Chemistry of Dental Care – Part 2
How to brush your teeth and how toothpaste works
The Chemistry of Dental Care – Part 3
Why fluoride is good for your teeth
See similar articles by Klaus Roth published in ChemistryViews Magazine