The Difficult Birth of a Champagne Bubble
After the turbulence due to pouring subsides, we are confronted with a roughly half-filled glass. Admittedly some of the CO2 departs unnoticed from the supersaturated solution by passing directly into the gas phase via the surface of the liquid, but some of it rewards us in its phase transition with a highly cherished spectacle: in certain places, on the walls of the glass, but also directly within the sparkling wine itself, tiny bubbles form, at regular intervals and seemingly out of nothing — bubbles that slowly rise, growing larger in the process, until they finally burst when they reach the surface.
1. Scientific Basis for the Fascinating Bubble Dance
The driving force behind bubble formation is the energy difference ΔG between a solution supersaturated with CO2 and one that is merely saturated [9]. The system is fundamentally striving to achieve an equilibrium state, so it releases CO2 until the saturation point is reached. Here, and in all the considerations that follow, we assume for the sake of simplicity that after the bottle is opened, the gas phase above the sparkling wine consists of pure CO2 at atmospheric pressure. That being the case:
ΔG = Gsat – Gsupersat = – RT lnδ (6)
where R is the ideal gas constant, T is the absolute temperature, and δ = (csupersat/csat), the supersaturation factor, which for a typical bottle of champagne immediately upon opening is about 5.5.
Now let us assume that a tiny microbubble is to be formed, with a volume Vmb = (4/3)πr3. This would of course slightly reduce the supersaturation of the champagne, which is to say the free energy of the system (champagne + microbubble) would also be reduced. If Vm is the molar volume for CO2 (22.4 L under standard conditions for an ideal gas), then
Δgmb = – (4πr3/3Vm) RT lnδ (7)
On the other hand, formation of the microbubble means that a new surface is created, a process that consumes energy, so the free energy of the system (champagne + microbubble) increases. The energy required is the product of the surface area Smb = 4πr2 times the surface tension γ. Taken together, for the formation of one tiny microbubble:
Δgmb = – (Vmb/Vm) RT lnδ + Smb γ = – (4πr3/3Vm) RT lnδ+ 4πr2 γ (8)
The first term, which is negative, encourages bubble formation, whereas the second, which is positive, acts against it.
With a small bubble radius, Δgmb initially increases, since the quantity of gaseous CO2 is relatively small while the surface area is relatively large. As the bubble grows larger, the first (negative) r3-term increases more rapidly, until overall Δgmb again starts to decrease. It therefore passes through a maximum at what is called the critical bubble size rc (see Fig. 3).
The equations look more challenging than they really are. To solve for the maximum, one need only differentiate Eq. (8) according to r, and then set the first derivative to zero, finally solving for r.
rc = 2 γ Vm /(RT lnδ) (9)
Eq. (9) leads to a critical radius for a champagne bubble of rc = 0.6 µm at a positive pressure of 5.5 atm and γchampagne = 0.05 N/m. It can be shown that spontaneous formation of such critical CO2 bubbles would require a CO2 pressure greater than 1000 atm [10].
.gif)
Figure 3. Thermodynamics and kinetics of bubble formation.
In Other Words
A CO2 bubble visible to the naked eye can only form in supersaturated sparkling wine from a microbubble with the critical size. Only bubbles with a radius of ca. 1 µm can increase in size by the incorporation of additional CO2. But there can be no spontaneous, homogeneous bubble formation in sparkling wine or champagne under ordinary circumstances. Since microbubbles cannot form spontaneously, the help of a suitable surface is required (see Fig. 6).
Bubble formation can occur only on a solid surface: i.e., heterogeneously. It has also been shown experimentally that, even at high supersaturation, bubbles will not form on smooth hydrophobic or hydrophilic surfaces [11]. This actually came as no surprise, since it has long been known that, with beverage glasses, bubbles in sparkling wines form preferentially at scratches on the interior glass surfaces. Glass manufacturers take advantage of this phenomenon in a creative way by deliberate roughening of the glass (with lasers or simply by scratching) to produce so-called Moussier points that help create especially attractive effervescent displays.
.gif)
Figure 4. a) Heterogeneous bubble formation at small depressions (scratches) in a champagne glass and b) on dust particles or small cotton fibers. The length of time between photographs here is 200 ms, but only 1 ms elapsed between photos 5 and 6. The bar in each case represents a length of 50 µm [12 – 14]. c) “String of pearls”. The bar again corresponds to a length of 50 µm.
Closer observation reveals that bubble formation begins not at peaks or ridges, but in recesses or hollows on the solid surface (see Fig. 6). It is here that tiny air bubbles become entrapped, which due to the slight curvature of the interface possess a radius exceeding the critical value. Under these conditions, CO2 can diffuse into the air bubbles, causing them to grow and eventually become detached, although a small gas residue remains behind so the game can begin anew.
Especially with a champagne glass, one frequently observes that somewhere in the midst of the liquid there appear to be bubble “fountains” out of which, with clockwork regularity, tiny bubbles materialize and then ascend, growing larger as they do so. Here again, what is occurring is heterogeneous bubble formation, but this time on air bubbles entrapped within cellulose fibers — cylindrical, ca. 100 µm long tubules with a diameter of 10 – 20 µm. The fibers may have come from dust particles, but most commonly the source will be the dish towel used to dry the glass. Capillary forces cause champagne to penetrate such tubules and wet their hydrophilic inner surfaces. Since liquid is invading from both ends, tiny air bubbles are entrapped, into which CO2 from the supersaturated wine can diffuse. The result is an increase in volume, until ultimately, from one of the ends, a tiny bubble separates and begins to rise. With a given geometry for the cellulose tubule, and at constant pressure, bubbles arise with almost clockwork regularity producing the treasured “string of pearls” effect.
2. The Inexorable Ascent of a “Champagne Pearl”
In water (density ρw =1.00 g/mL), the buoyant force acting on a tiny bubble (diameter d) is
FA = m g = (π/6) ρw g d3 (10)
where g = 9.81 m s–2, the acceleration due to earth’s gravity. To be precise, this force is a function of the density difference (rw – rg) between the gas and water, but the density of a gas (e.g., carbon dioxide = 0.00022) is negligibly small compared to that of water. The gas bubble’s ascent is retarded by surrounding water, since water molecules must be forced aside as the bubble rises. This is nothing more than a case of friction, and the corresponding frictional force Ff is given by Stokes’ Law:
Ff = 3π η d vA (11)
where η is the viscosity of water and vA is the ascent velocity of the gas bubble.
After a short period of time, a constant ascent velocity vA is established, because then the buoyant force and the opposing frictional force have become equally large. At this point:
vA = g ρw d2/(18 ηw) (12)
Introducing numerical values for g, ρw, ηw (ηw = 1.5 10–3 kgm–1s–1) in Eq. (12) leads to:
vA = 3630 d2 cm/s (13)
For a gas bubble with a diameter of 0.1 mm, Eq. (13) indicates an ascent velocity vA = 3.6 mm/s.
So far we have considered only a single tiny bubble with a constant diameter. Once a critical bubble has formed in the sparkling wine, it ascends, and during its climb it grows steadily larger as CO2 diffuses in from the supersaturated liquid surrounding it (Fig. 5). The increase in the number of CO2 molecules within the bubble as a function of time (dN/dt) is a function of the CO2 saturation factor, and is proportional, as indicated in Eq. (4), to the diffusion coefficient D and to the surface area of the bubble A [15], where the additional quantity w is a function of a series of flow parameters. Under a specified set of conditions, w can be calculated using three dimensionless numbers: the Sherwood, Peclet, and Reynolds numbers. For an (American) calculation based on the example of Budweiser Beer, see [16].
dN/dt = w D (δ – 1) A (14)
Increasing the number of particles N causes an increase in volume, and according to a form of the ideal gas law pV = NkT (where k = the Boltzmann constant):
dN/dt = (P/kT) (dV/dt) = (P/kT) 4πr2 (dr/dt) (15)
From Equations (14) and (15) it follows that, in general, for the change with time of the radius of a growing bubble:
(dr/dt) = w D (δ – 1) (kT/P) (16)
This differential equation has the solution:
r = r0 + w D (δ – 1) (kT/P) t (17)
The radius of a rising champagne bubble in a supersaturated solution increases linearly with time, with typical values of 350 – 400 µm/s.
According to Eq. (13) one should expect a quadratic relationship between the ascent velocity of a bubble and its radius (or diameter). From this it directly follows that the distance between two successive bubbles in a “string of pearls” increases during the ascent.
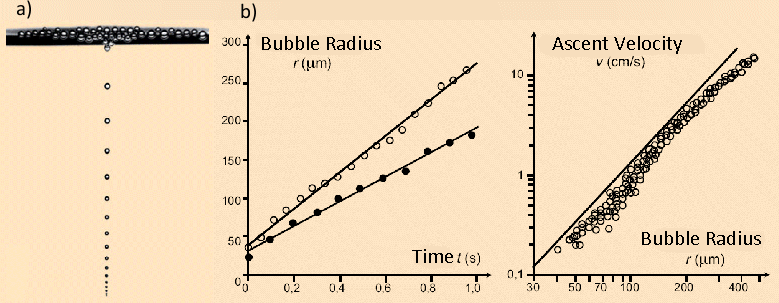
Figure 5. a) Champagne pearls or beads rising in a glass. b) The radius of each bead increases linearly on the way to the surface, since more CO2 from the supersaturated solution migrates into the interior of the bubble. As a consequence, the rate of ascent of bubbles increases nearly as the square of the radius, as does the separation between successive bubbles.
Figure 5 shows that, according to measurements on ascending bubbles, clear deviations occur from a linear relationship between radius and time, and also from a quadratic relationship between radius and ascent velocity. It is sobering to have to concede that our elementary approach has failed to fully characterize the complex behavior of ascending and expanding gas bubbles. Fluid mechanics, in fact, reveals that the frictional drag on bubbles in a liquid is heavily dependent on the velocity itself [17, 18].
Furthermore, gas bubbles are not rigid spheres, but can change in shape as they ascend, and they do so to some extent in an oscillating fashion [19]. Finally, champagne is (fortunately!) not distilled water, but instead contains a significant concentration of surfactant molecules (mainly proteins). This can lead to retardation of the ascent velocity with increasing radius or height (the Marangoni effect) [20].
If one gazes dreamily for too long at the display of pearls, and forgets to imbibe, it will be observed that over time the bubble diameter decreases, and these bubbles ascend more slowly. The effect has already been described by Eq. (17), according to which, with decreasing supersaturation, δ, the radii of the bubbles becomes smaller, so their volume during ascent grows more slowly.
Other beverages that are supersaturated with CO2, such as soda water and beer, differ in their characteristics with respect to median size and ascent velocity of CO2 bubbles (see Fig. 6). The differences can be seen at a glance between seltzer (see Fig. 6a), champagne (see Fig. 6b), and beer (Fig. 6c). Bubbles rise fastest (and are largest) in pure mineral water. Champagne contains natural surfactants that accumulate at interfaces between CO2 and the beverage, and thus — despite the higher pressure — retard diffusion of CO2 into the rising bubbles, and thus their growth. Champagne bubbles grow at ca. 350 – 400 μm/s at 20 °C (68 °F). Beer contains especially large amounts of surfactant proteins and hops components, and the CO2 pressure is lower than in champagne, so CO2 bubbles in beer are especially small, and compared to champagne they ascend significantly more slowly (100 – 150 μm/s) [22].
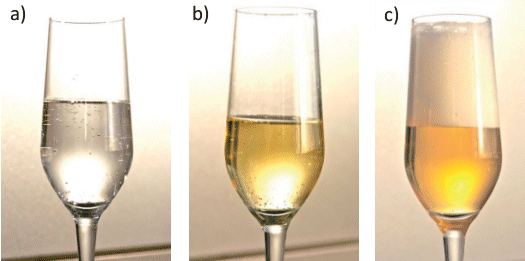
Figure 6. Seltzer water (a), sparkling wine(b) – or beer (c)?
3. Why Does Sparkling Wine And Champagne Taste So Good?
Despite all these profound physical-chemical reflections regarding the effervescence of sparkling wine or champagne, we certainly should not forget the sensuous pleasure, which is determined by the quality of the starting materials and competence of the processing. The various base wines utilized are relatively light, and assembled by adept hands. The second fermentation is initiated by adding liqueur de tirage — a small amount of yeast, sugar, wine, and “must” — which not only causes a generation of CO2 and an increase in the alcohol content by 1–1.5 %, but because of the added yeast (a living organism!), new aromatic substances and flavoring agents are also created, and some of those already present are decomposed [23].
Apart from intensity changes in various aromatic attributes (see Fig. 7), the second fermentation accomplishes, for example, significant decomposition of 2-aminoacetophenone, present in many prematurely harvested base wines, which can lend the sparkling wine an undesirable “furniture polish” tinge [24]. In this context it is of special interest that the bubbles bursting at the surface spray an aerosol into the surrounding gas, through which not only nonpolar aromatic substances are conveyed to the nose of the savorer, but also surfactant substances. Preliminary studies have detected a multitude of such compounds, only a small fraction of them so far identified [25].
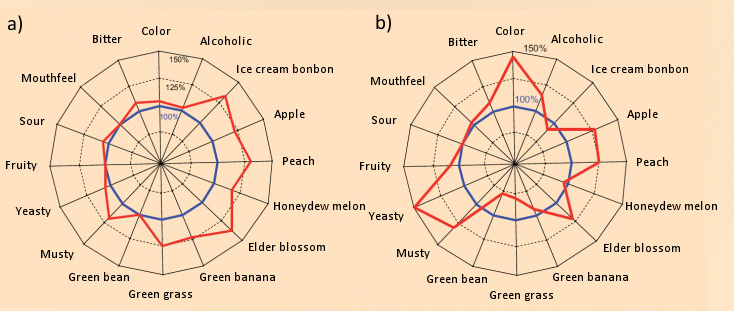
Figure 7. Second fermentation and the aroma of a champagne.
Second fermentation in the bottle or in a pressure tank leads to two major chemical changes: an increase of ca. 1 – 1.5 % in alcohol content, and formation of a considerable amount of CO2, which at an internal pressure of 5 atm, is largely in solution. The above results are taken from an impressive study by Ganss et al., conducted at the Dienstleistungszentrum Ländlicher Raum (DLR) in Neustadt an der Weinstraße (Service Center, Rural Region, located in Neustadt on the Weinstrasse) [23, 24].
(a) In order to examine the influence on aroma exclusively of increased content of alcohol and CO2, 1.5 % pure alcohol and CO2 were added under pressure to a base wine (Riesling). 18 trained examiners rated individual aspects of the base wine (sensory intensity 100 %), and then of freshly poured samples of the fortified and carbonated “sparkling wine”. A significant intensification was recognized with respect to nearly all the aromatic “tonal qualities” due to CO2 bubbles bursting at the surface and transporting aromatic components to the surrounding air and the examiner’s nose. Thus, CO2 bubbles greatly intensify the aroma.
(b) Compared to the base wine (sensory intensity 100 %), “sparklification” of that same base wine with yeast led to further changes in aromatic profile. Relative to the base wine, the “green tones” (green bean, green grass) were suppressed, whereas the “fruity tones” (honeydew melon and peach) became more intense. These changes may be due to a significant reduction in the concentration of nonanal (green grass) and an increase in the amounts of esters responsible for fruity tones (ethyl propionate, ethyl 3-methylbutyrate, and ethyl hexanoate).
The CO2 in sparkling wine is a treat not only to our senses of taste and smell, but also the sense of touch, because it produces a tingling or even a slight burning feel on the tongue. This can’t be merely a mechanical stimulation at the tongue’s surface as a consequence of the bursting bubbles, because one senses the tingling even when no bubbles are left on the tongue. This was confirmed by studies conducted in a pressure chamber, where the increased pressure ensures no bubbles could form, but a tingling sensation was still experienced on the tongue. In other words, there is more behind this phenomenon: the CO2 begins by dissolving in the mucous membrane and then actually penetrates the tongue tissue. There, the dissolved CO2 is hydrated to carbonic acid, which in turn activates neurons and causes the tingling or tickling sensation [26, 27]. The formation of carbonic acid from CO2 appears at first glance to be a simple chemical process. It may come as a surprise to learn, however, that what in many chemistry textbooks is portrayed as a straightforward equilibrium reaction in fact occurs only very slowly, in both directions. In the human body the reaction is greatly accelerated by one of the most efficient of enzymes: carboanhydrase. One molecule of carboanhydrase transforms into carbonic acid what may be a record-breaking 36 million CO2 molecules per minute [28, 29] !
As carboanhydrase increases the rate of formation of carbonic acid from CO2 dissolved in water by a factor of 1.4 x 108, it is among the most effective enzymes known [29]. Interestingly, the native enzyme (see Fig. 8, left) [30, 31] changes in geometry only a tiny bit upon binding to the hydrogen carbonate anion (see Fig. 8, right).
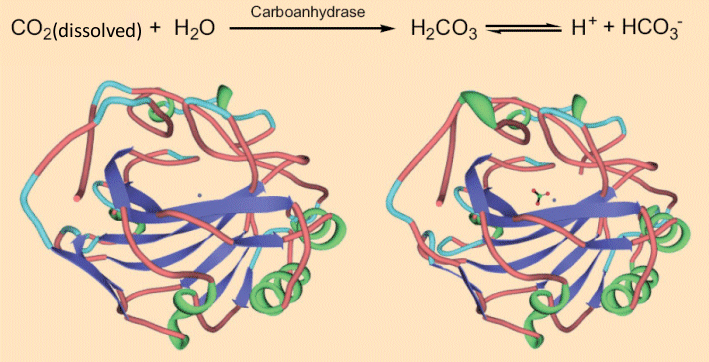
Figure 8. Carboanhydrase and the tickling of the tongue.
Two interesting experiments have proven the central role played by carboanhydrase in the enjoyment of champagne or sparkling wine. On one hand, if half the tongue is treated with a carboanhydrase inhibitor, a tingling sensation is no longer felt on this half of the tongue, but is still experienced on the other half. On the other hand, something known as the “champagne blues” is commonly experienced by climbers in the high Alps: upon descent from a climb, carbonated drinks including champagne taste flat and insipid, and beer has been described as tasting “like dishwater”. The explanation is simple: many climbers take a carboanhydrase inhibitor (acetazolamide) to prevent altitude sickness, and for a while they are then no longer able to sense the tingling of the tongue otherwise produced by carbonated beverages.
The quality of the perlage in champagne — that is to say the long-lasting formation of the smallest possible, slowly ascending CO2 bubbles — is an important criterion of overall quality for the connoisseur, although there is no relationship between bubble size and the number and nature of aroma and flavor components. Nevertheless, the production of bubbles that ascend and burst at the surface does result in a spray of aerosols the nose in turn inhales (see Fig. 9).
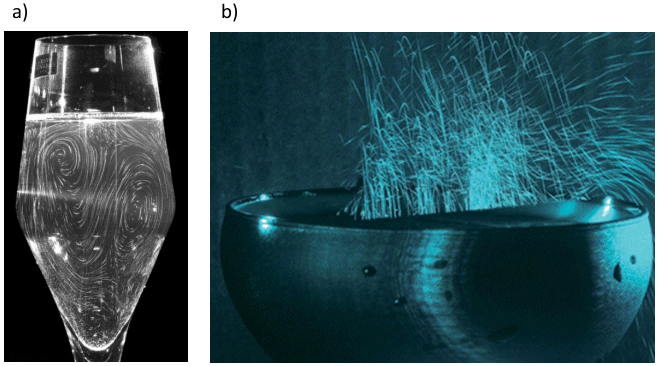
Figure 9. Thorough mixing and aerosol formation.
a) By careful injection of a fluorescein solution a short time after champagne has been poured into a tulip glass, and with the aid of a laser it becomes possible to see flow motion within a single plane. From this it can be seen that there is a continuous exchange between upper and lower liquid layers so that concentrations at the surface of aromatic components — crucial to the connoisseur’s pleasure — remain constant.
b)This photo, with an exposure time of 1 s, shows traces of the many microdrops flung into the air when CO2 bubbles burst at the surface. These microdrops function like a “hail Mary” in the champagne with respect to aroma components, especially the less volatile ones that otherwise would have absolutely no chance of reaching the epicure’s nose because of their very low vapor pressure.
Champagne Quickly Goes to Our Heads
A recent study by Fran Ridout at the University of Surrey in Guildford, UK, has confirmed what was already general knowledge: Champagne goes to the head very quickly. CO2 bubbles are behind this phenomenon as well. In Ridout’s study, 12 volunteers drank two glasses of champagne [32]. The precise quantity was adjusted so that all the subjects received the same amount of alcohol per kg of body weight. Half the subjects were given fresh champagne, and the other half a champagne that had previously been made carbonic acid-free with the aid of a swizzle stick.
After five minutes, the sparkling champagne had produced a blood alcohol content of 0.54 % and the flat champagne only 0.39 %. After 40 minutes the values were 0.70 % and 0.59 %, respectively. Apparently, bursting bubbles stimulate the walls of the stomach, leading to increased circulation, in turn causing more rapid alcohol uptake by the blood. This result clearly confirms experience: sparkling wine and champagne do go to the head more quickly.
The famed French gourmet Jean Brillat-Savarin captured it in a nutshell:
“Burgundy makes you think of foolish things,
Bordeaux makes you talk about them,
and Champagne makes you do them.”
4. What to Do With an Opened Bottle of Champagne?
Often you might wish to preserve opened bottles of expensive sparkling wine or champagne for later enjoyment without the wine going flat. It has frequently been suggested that one should insert a spoon (preferably silver) into the bottle, with the handle down. Incredulous, we wonder: how can a spoon hanging in a bottle retard the release of carbon dioxide?
Many sommeliers swear by the silver spoon, but every attempt to confirm its effectiveness has been a failure. Most testers confirm that if champagne is left open in a refrigerator, by the next day — or even the day after that — it will have barely lost any of its freshness, with or without a silver spoon [33]. Richard Zare and a small group of his friends at Stanford University, USA, devoted themselves to this issue in what was a self-financed project. Thus, five separate bottles of a premium California sparkling wine were submitted to blind tasting after various treatments as follows [34]:
Bottle1: Shortly before the blind tasting it was removed from the refrigerator and opened, and a portion was poured into a glass.
Bottle 2: This one was opened 26 hours earlier, the appropriate sample was poured into a glass, and the bottle was then placed — uncovered — back in the refrigerator.
Bottle 3: Again opened 26 hours previously, with a sample poured into a glass, and then placed uncovered back in the refrigerator, but this time with a silver spoon stuck in the neck of the bottle.
Bottle 4: Opened 26 hours previously, a sample poured into a glass, and the bottle returned uncovered to the refrigerator, but with a stainless steel spoon stuck in the neck of the bottle.
Bottle 5: Opened 26 hours previously, a sample poured into a glass, but the bottle this time recorked before being put back in the refrigerator.
Despite obvious statistical uncertainty, and a motley crew of untrained testers, clear declarations still resulted:
First Place
Bottle 2, which was left open overnight in the refrigerator, had preserved the freshness of the sparkling wine the best. In fact, the subjects found that it tasted even better than the freshly opened bottle!
Second Place
Bottle 1, opened immediately before the tasting, came in second.
Third Place
Bottles 3 and 4, stored open overnight in the refrigerator, but with a spoon in the neck of each bottle, tied for third place, but no distinction could be made on the basis of the metal from which the spoon was made.
Fourth Place
The recorked Bottle 5 came in last. In the opinion of the judges, returning a cork to the bottle was the surest way to cause the wine to become flat and less tasty.
The result is clear: an opened sparkling wine or champagne bottle should be carefully — i.e., without shaking — returned, unsealed, to the refrigerator. It is important to refrain from putting the cork back in, since that can cause numerous small cork fragments to fall into the liquid, thereby facilitating thereafter a continuous formation of bubbles. The only idiot-proof way to keep champagne from becoming flat, irrespective of Henry’s Law, thermodynamics in general, or the formation of strings of pearls, is simply to drink it!
So cheers!
References
[9] K. Meyer, Wiss. Fortschritt 1985, 35, 136.
[10] P. M. Wilt, J. Coll. Interf. Sci. 1986, 112, 530. DOI: 10.1016/0021-9797(86)90122-0
[11] W. L. Ryan et al., J. Coll. Interf. Sci. 1993, 157, 312. DOI: 10.1006/jcis.1993.1191
[12] G. Liger-Belair et al., Langmuir 2004, 20, 4132. DOI: 10.1021/la049960f
[13] G. Liger-Belair et al., J. Phys. Chem B, 2005, 109, 14573. DOI: 10.1021/jp051650y
[14] G. Liger-Belair et al., J. Phys. Chem B, 2006, 110, 21145. DOI: 10.1021/jp0640427
[15] H.-J. Schlichting, C. Ucke, Physik Unserer Zeit 1993, 24, 231. DOI: 10.1002/piuz.19930240512
[16] Y. Zhang, Z. Xu, Elements, 2008, 4, 47. www.elementsmagazine.org
[17] N. E. Shafer, R. N. Zare, Physics Today 1991, 10, 48. DOI: 10.1063/1.881294
[18] Y. Zhang, Z. Xu, Elements 2008, 4, 47. www.elementsmagazine.org
[19] G. Liger-Belair et al., Langmuir 2002, 18, 1294. DOI: 10.1021/la0115987
[20] G. Liger-Belair et al., J. Agric. Food Chem. 2005, 53, 2788. DOI: 10.1021/jf048259e
[21] K. Roth, Chem. Unserer Zeit 2006, 40, 338. DOI: 10.1002/ciuz.200600398
[22] R. N. Zare, J. Chem. Educ. 2005, 82, 673. DOI: 10.1021/ed082p673
[23] S. Ganß, S. Wolz, H.-G. Schmarr, U. Fischer, DLR Rheinpfalz Aktuell 2006, 39.
[24] H.-G. Schmarr et al., J. Chromatogr., A 2007, 1150, 78. DOI: 10.1016/j.chroma.2006.09.001
[25] G. Liger-Belair et al., Proc. Nat. Acad. Sci. USA 2009, 106, 16545. DOI: 10.1073/pnas.0906483106
[26] E. Carstens et al., Food Qual. Pref. 2002, 13, 431. DOI: 10.1016/S0950-3293(01)00067-2
[27] J.-M. Dessirier et al., Chem. Senses 2000, 25, 277. DOI: 10.1093/chemse/25.3.277
[28] R. Ludwig, A. Kornrath, Angew. Chem. 2000, 112, 1479. 3.0.CO;2-W”>DOI: 10.1002/(SICI)1521-3757(20000417)112:8<1479::AID-ANGE1479>3.0.CO;2-W
[29] D. N. Silverman, S. Lindskog, Acc. Chem. Res. 1988, 21, 30. DOI: 10.1021/ar00145a005
[30] K. K. Kannan et al., Ann. N. Y. Acad. Sci. 1984, 429, 49. www.nyas.org
[31] K. K. Kannan et al., J. Mol. Biol. 1994, 241, 226. DOI: 10.1006/jmbi.1994.1491
[32] A. Coghlan, New Scientist 2001, Dec. 22, 7. www3.surrey.ac.uk/news
[33] H. Speer, Suffolk Food & Drink 2009, January, 88. www.champers.net/suffolklifechampers.pdf
[34] www.stanford.edu/dept/news/pr/94/941221Arc4008.html
Author
Professor Klaus Roth, Freie Universität Berlin, Germany.
The article has been published in German in:
and was translated by W. E. Russey.
► Part 1 – Only Chemistry Can Be This Tingling
► Part 2 – Opening the Bottle and Choosing the Glass
Other articles by Klaus Roth published by ChemistryViews magazine:
-
- In Espresso — A Three-Step Preparation
Klaus Roth proves that no culinary masterpiece can be achieved without a basic knowledge of chemistry
DOI: 10.1002/chemv.201000003 - In Chemistry of a Hangover — Alcohol and its Consequences
Klaus Roth asks how a tiny molecule like ethanol can be at the root of so much human misery?
DOI: 10.1002/chemv.201000074 - In Chocolate — The Noblest Polymorphism
Klaus Roth proves only chemistry is able to produce such a celestial pleasure
DOI: 10.1002/chemv.201000021 - In The Chemist’s Fear of the Fugu
Klaus Roth shows the chemist’s fear of the fugu or pufferfish extends as far as the distinctive and intriguing poision it carries
DOI: 10.1002/chemv.201000104
- In Espresso — A Three-Step Preparation





Very interesting article. I also came across an entry in a 1965 publication ‘The Dictionary of Drink’ by Oscar A. Mendelsohn. Sparkling: “The general English term for a wine charged with carbon dioxide gas (q.v.). It is never applied to the finest of such wines, champagne. It is used, perhaps unfortunately, for both the naturally-sparkling wines made by the champagne process (q.v.) such as sparkling burgundy, hock, moselle (and cider) and for artificially carbonated beverages. The finest burgundies, hocks, etc., are never used for sparkling wines but the products may be very agreeable nevertheless. In their own countries these sparkling wines are comparatively inexpensive but as non-wine-producing countries unfairly levy the same tax on them as on champagne, they are not usually good value. It has long been recognized that the true champagne process produces a longer and more controlled sparkle than does carbonation effected by pumping in the gas. This is probably due to the actual chemical combination of the carbon dioxide gas with the alcohol in champagne-process wine to form the unstable compound ethyl pyrocarbonate. In carbonated wines the gas is merely dissolved and escapes more rapidly. See also Sparkling Wine. I would appreciate a comment from Klaus Roth.