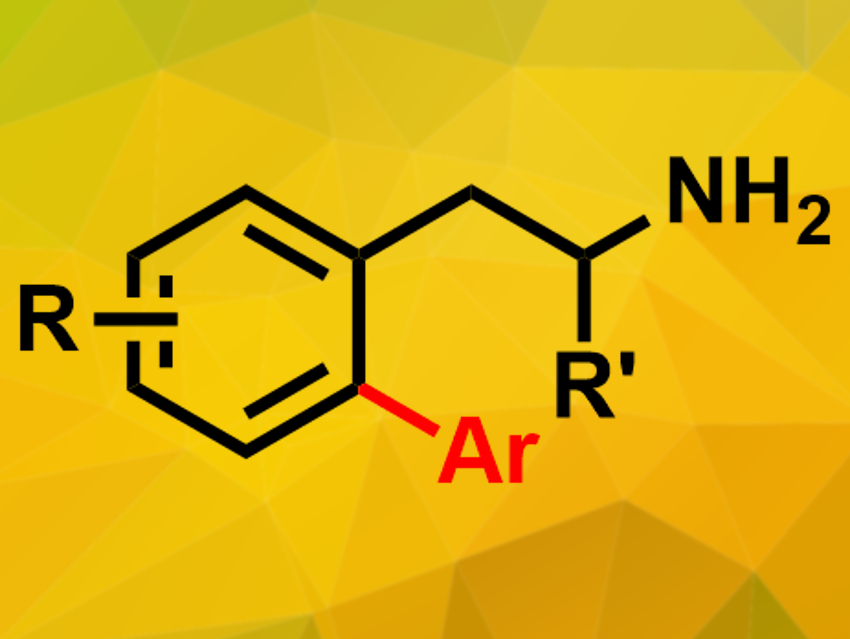
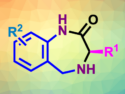
2-Phenylethylamines are structures found in several pharmaceutically active molecules, e.g., in amphetamines. Modification of this type of coumpound could lead to new drug candidates. Ortho-C–H activations at the phenyl substituent, however, usually require added directing groups to steer the reactivity.
Yuzhen Gao, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, China, Gang Li, Fujian Institute of Research on the Structure of Matter and University of the Chinese Academy of Sciences, Beijing, and colleagues have developed a palladium-catalyzed C(sp2)–H arylation of free 2-phenylethylamines (product pictured). The existing amines are used as directing groups in this approach. The team used Pd(OAc)2 as a catalyst and an acetate-protected amino acid as a ligand in the presence of triflic acid and Ag2CO3. Under these conditions, free 2-phenylethylamines and aryl iodides react to give the desired products in good yields.
According to the researchers, the free amine coordinates the palladium catalyst to give a six-membered palladacycle. Thus, it acts as the directing group to ensure ortho selectivity. The method could be useful in pharmaceutical chemistry.
- Ligand Promoted, Palladium-Catalyzed C(sp2)–H Arylation of Free Primary 2-Phenylethylamines,
Yongzheng Ding, Shuai Fan, Xiaoxi Chen, Yuzhen Gao, Shangda Li, Gang Li,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b01411