Hückel’s Rule
Aromatic molecules, which have cyclic delocalized π-electron systems, are particularly stable and have distinct magnetic and electronic properties. Hückel’s rule can be used to determine whether a particular cyclic molecule with a delocalized π-system will have aromatic or antiaromatic properties. Rings with [4n + 2] π-electrons are aromatic and rings with 4n π-electrons are antiaromatic, with n being a positive whole number. These two types of molecules have opposing magnetic properties. In the presence of a magnetic field, the circulating π-electrons produce a ring current that induces a second magnetic field. In aromatic molecules, the induced field opposes the external field inside the ring, while antiaromatic molecules have the opposite magnetization. This effect can be observed using NMR spectroscopy.
 Hückel’s rule is usually applied to small, planar molecules. It is not clear whether it also holds for large systems or whether there is a general size limit for aromatic behavior. So far, there had been no known aromatic rings with more than 62 π-electrons.
Hückel’s rule is usually applied to small, planar molecules. It is not clear whether it also holds for large systems or whether there is a general size limit for aromatic behavior. So far, there had been no known aromatic rings with more than 62 π-electrons.
Aromatic Nanorings?
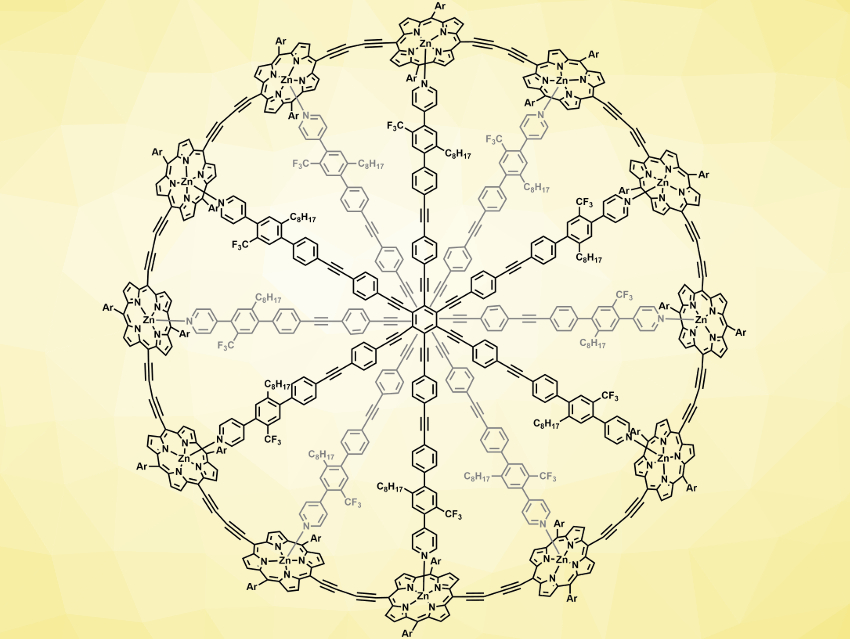
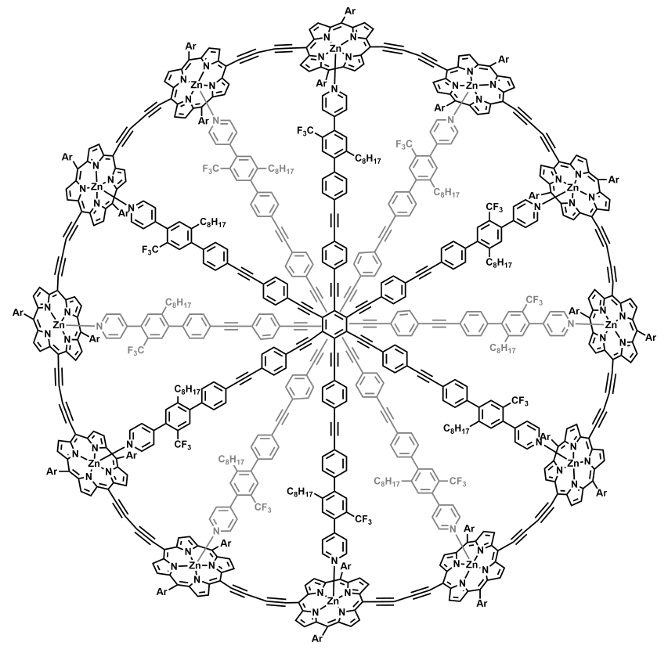
Harry L. Anderson, University of Oxford, UK, and colleagues have studied porphyrin nanorings with up to 162 delocalized π-electrons. The team created rings made from zinc porphyrins and -C≡C- groups, using a central organic structure with six-fold symmetry. They varied the number of π-electrons and the size of the rings by changing the number of porphyrin and -C≡C- units. In the largest ring (pictured), two of the central six-armed templates stacked to form a ring with twelve porphyrin units. The systems were studied using 1H, 13C, and 19F NMR spectroscopy.
The team found that the delocalization of π-electrons extends around the nanorings, which have circumferences of up to 16 nm. The magnitude of the ring currents changes the with oxidation state of the porphyrin units. Despite the comparatively large size of the systems, Hückel’s rule correctly predicts the direction in the ring currents occurring in the nanorings.
- Global aromaticity at the nanoscale,
Michel Rickhaus, Michael Jirasek, Lara Tejerina, Henrik Gotfredsen, Martin D. Peeks, Renée Haver, Hua-Wei Jiang, Timothy D. W. Claridge, Harry L. Anderson,
Nat. Chem. 2020.
https://doi.org/10.1038/s41557-019-0398-3