From the beginning, there have been many legends and stories about the discovery of Prussian blue in 1706. The process for making it, which was initially kept secret and was highly lucrative, was given away in 1724. What happened?
3 Dippel’s Quarrelsome Nature Almost Destroyed the Business
Instead of concentrating on the economic success of the new product, the pugnacious Johann Konrad Dippel could not stop himself from writing another ultra-pietistic polemic entitled “Impartial Thoughts on a So-Called Swedish Theological Short Report about Pietists” under his pseudonym of Christianus Democritus. In this pamphlet, published in 1706, he grumbled against a Lutheran theologian at the University of Greifswald. Because northern Mecklenburg-Western Pomerania belonged to Sweden after the Thirty Year War, King Karl XII of Sweden was offended. The subtitle of the polemic, “About the Brutality and Illegality of Religious Compulsion”, did not really demonstrate Dippel’s political finesse, since the distance between Berlin and the Swedish troops was only 150 km and the word of the Swedish ambassador had some influence in the Prussian court.
In February 1707, Dippel was arrested by the Prussian police because of his insult to the Swedish king. After one week, he was released on bail, only to be threatened with another arrest due to pressure from the Swedes. Dippel’s only option was to flee hastily toward Holland, dressed as a Swedish officer.
Johann Jacob von Diesbach was suddenly alone. He had become an experienced experimenter, but the economic success of a product also requires good marketing. Diesbach could not do this himself and urgently needed a product manager. Luckily, he knew a suitable person who had already acquired some experience with chemical laboratory practice in his lab: the subdirector of the Berlin High School of the Gray Cloister.
 |
|
Johann Konrad Dippel |
4 Johann Leonhard Frisch (1666–1743)
Diesbach and Frisch joined forces and divided responsibilities: Diesbach managed production, Frisch took over sales. Frisch was a natural salesman who successfully made the blue pigment popular among European artists. It was helpful that it only cost one-tenth the price of its competitor, ultramarine.
On March 31, 1708, Frisch wrote, “My paint has not only returned the expenses I spent on it, but also some excess.” This was something of an understatement because documented property purchases indicate more than “some excess.”
4.1 First Publication Increases Popularity
In addition to his work as a teacher, Frisch was a renowned student of language and a naturalist. He wrote a German-Latin dictionary, as well as a comprehensive work about the insects and birds of Europe. He was a member of the Royal Prussian Academy of Sciences. The Academy planned a series of scientific publications, and in 1710, Frisch wrote a short article entitled “Notitia Cœrulei Berolinensis” for the first volume, introducing the term “Berlin blue” (Prussian blue) to the scientific literature. (see Fig. 6) [14,15].
 |
| Figure 6. First publication about Prussian blue. |
As a promotion, Johann Leonhard Frisch originally wanted to accentuate the title of his article by printing it in an ink made of Prussian blue. The printer hired for the job, Johann Wessel, was, at least according to Frisch, the “most inept we have, if he is asked to do anything out of the ordinary.” Frisch gave up on this notion, which we have rectified in his honor.
Frisch did not give away anything about the production process in this article. The publication was essentially an advertisement in which the pigment was praised in the highest terms. As is usual in advertisement, it was vastly exaggerated. Frisch claimed that Prussian blue was stable toward burnt lime, which was definitely not true. However, this did not matter, because false advertisements were just as effective at that time as they are now. Prussian blue, thus, achieved a firm footing on the palettes of European painters (see Fig. 7).
 |
| Figure 7. The oldest painting in which Prussian blue has been detected is Pieter van der Werff’s “Entombment of Christ” from 1709, which hangs in the Picture Gallery of Sanssouci in the park of Sanssouci Palace in Potsdam. Prussian blue was used in Mary’s cloak and in the sky. (Image source: Prussian Palaces and Gardens Foundation Berlin-Brandenburg/Daniel Lindner). |
5 The Year 1724: Betrayal
It was predictable that such a lucrative production process could not be kept secret forever. (Though some have managed to keep an industrial secret for decades, like Henry Bessemer with his bronze powder [16].) The first privately sent recipes for Prussian blue were already floating around [17]. The guessing game ended abruptly in 1724, when the complete production process was published in detail in the Philosophical Transaction of the Royal Society [18] (see Fig. 8):
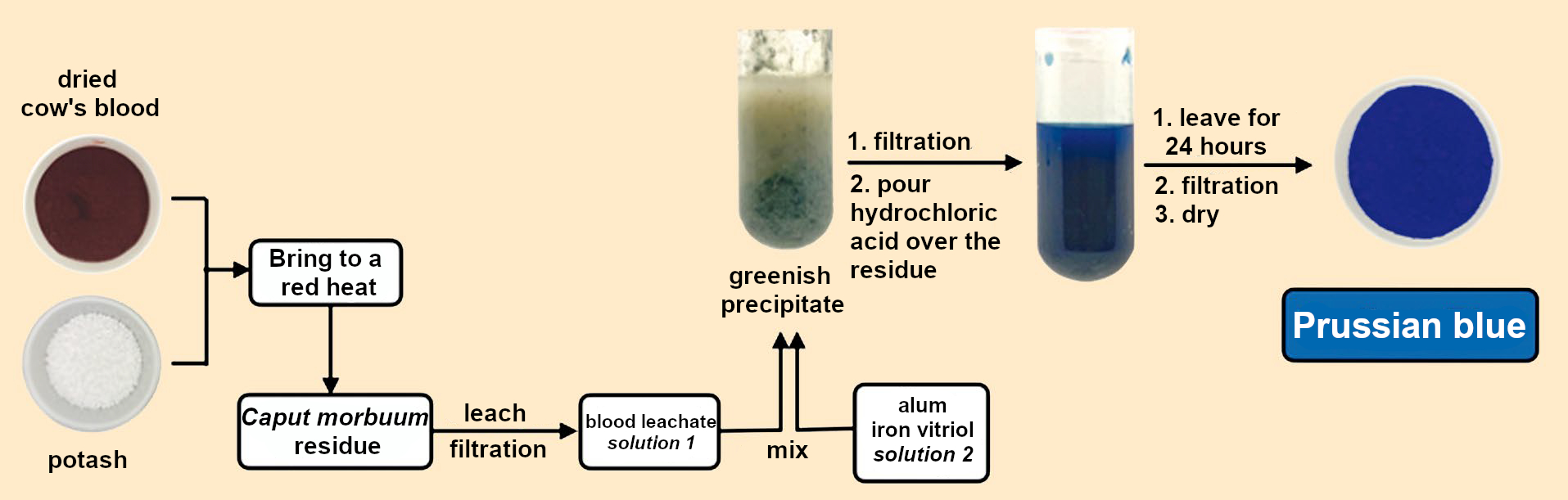 |
|
Figure 8. The recipe revealed. |
Combine 124 g each of pulverized potash and dried cow’s blood. Place in a crucible with a lid and heat until it glows brightly and flames emerge from the crucible lid. After cooling, the black residue is decocted with 1.8 L water for half an hour, filtered, and the residue leached out until the filtrate is flavorless (!). All filtrates are combined and concentrated to 1.8 L (solution 1, see Fig. 8).
A boiling solution of 31 g iron vitriol is combined in a large pot with a boiling solution of 248 g alum in 1.8 L water each (solution 2, see Fig 8). (Vitriols are hydrated sulfates of mainly divalent metals, such as iron(II) sulfate heptahydrate or FeSO4•7 H2O.) To this is added boiling solution 1. A malachite-green precipitate is formed. After cooling, it is filtered through a linen cloth. The solid obtained is placed in a pot and 60–90 mL hydrochloric acid is poured over it and stirred. The most beautiful blue color appears immediately. After standing overnight, the supernatant hydrochloric acid is decanted and the remaining solid is rinsed with rainwater and stirred with a spatula. After settling, the liquid is decanted again and fresh water added. This is repeated until the water poured off has no taste (!). The blue solid is then filtered out and dried.
This procedure can still be used today to produce Prussian blue from dried cow’s blood, potash, alum, and iron vitriol. If this process is compared to those used by Dippel and Diesbach in 1706 to make Florentine lake, animal oil, and hartshorn (see Fig. 1 and 4 in Part 1), chemical similarities to the production of Prussian blue become apparent (see Fig. 8). It is not possible to draw an unambiguous conclusion about what went wrong for Diesbach in 1706, however.
Beginning in 1724, the whole world was able to produce Prussian blue and the alchemists of Berlin lost their monopoly. Within a short time, Prussian blue factories were built in many countries and given the enormous economic damage to the entrepreneurs in Berlin, the question arises: Who revealed the recipe?
References
[14] J. L. Frisch (Anon.), Notitia Cœrulei Berolinensis, Miscellanea Berolinensia ad incrementum Scientiarum, 1710, 1, 377.
[15] A. Kraft, “Notitia Cœrulei Berolinensis nuper inventi”: On the 300th Anniversary of the First Publication on Prussian Blue, Bull. Hist. Chem. 2011, 36, 3–9.
[16] K. Roth, Sir Henry’s Secret Pot of Gold, ChemistryViews 2019. https://doi.org/10.1002/chemv.201900053
[17] A. Kraft, Wege des Wissens: Berliner Blau, 1706-1726, Mitteilungen der Fachgruppe Geschichte der Chemie der Gesellschaft Deutscher Chemiker 2012, 22, 3–19.
[18] J. Woodward, Praeparatio Caerulei Prussiaci Ex Germania Missa ad Johannem Woodward, M. D. Prof. Med. Gresh. R. S. S., Phil. Trans. R. Soc. 1724/1725, 33, 15–17.
The article has been published in German as:
- Berliner Blau – Entdecker und Verräter,
Klaus Roth,
Chem. unserer Zeit 2021, 56, 34–49.
https://doi.org/10.1002/ciuz.202100033
and was translated by Caroll Pohl-Ferry.
Prussian Blue: Discovery and Betrayal – Part 1
Around 1700, Berlin was a colorful center of innovation – a scene that led to the discovery of the century: Prussian blue
Prussian Blue: Discovery and Betrayal – Part 3
Who leaked the secret recipe for Prussian blue?
Prussian Blue: Discovery and Betrayal – Part 4
How was Prussian blue really discovered—with luck or a stroke of genius?
Prussian Blue: Discovery and Betrayal – Part 5
Solving the case of the discovery of Prussian blue
See similar articles by Klaus Roth published on ChemistryViews.org




This is very interesting. Thanks for sharing! People should know how to make this substance to keep around in case of emergency aka nuclear disaster. What would help even more is an explanation of how it’s used as a medicine aka decorporation compound to detoxify cesium 137 and thallium out of the human body as well as a short discussion of other decorporation compound that may be able to mitigate/remove strontium 90 and cobalt 60 etc. in case of a nuclear disaster when the general public most likely will not have access to these compounds. Thank you.