From the beginning, many legends and stories have swirled around the 1706 discovery of Prussian blue. The highly lucrative and initially secret process was revealed in 1724, causing the Berlin alchemists to lose their monopoly.
After the London article, every halfway decently educated alchemist or chymist was able to produce Prussian blue. But how did Diesbach and Dippel come up with this recipe in the first place? Why dried blood? Why potash? Was it a stroke of genius, or a miracle sent from heaven?
8 Two Late Witness Reports about the Discovery of Prussian Blue
Diesbach and Frisch could have answered this question, but both remained silent and continued to produce Prussian blue in secret. Dippel, who was probably more qualified, was directly involved in the discovery, but could not talk because he was locked up in 1724, sentenced to a life term in prison on the Danish island of Bornholm. A number of second-hand versions of the story floated around until Georg Ernst Stahl, founder of phlogiston theory and personal physician to the Soldier King, introduced his version of the discovery of Prussian blue because it would be worth the effort “to capture the first experiment, how it is said to have happened, in a permanent memorial to the event.”
8.1 The Discovery of Prussian Blue, According to Georg Ernst Stahl (1731)
In his 1731 book “Experimenta, Observtiones, Animadversiones, 300 Numero Chymicae et Physicae,” Stahl described the discovery as follows [23]:
“A paint merchant named Diesbach, who, in order to make Florentine lake, would mix a decoction of cochineal with alum and some iron vitriol, and then precipitate it with fireproof alkali, once didn’t have any alkali and thus borrowed from Dippel, in whose laboratory he was working, some potash, over which this chymist had distilled his animal oil several times. The lake that was precipitated with this alkali, instead of being red, was a very beautiful blue. Dippel, whom he notified of this phenomenon, noted that this arose from the nature of his alkali and tried to produce this effect by imparting this property to other alkali through a simplified process. The experiments that he undertook were successful, and from that time on, the discovery of Prussian blue was a famous thing.”
Stahl was no eyewitness. He was summarizing what was being said in scientific circles at the time. No one noticed that his account differed from those of Woodward and Brown [18,19]. In those procedures, absolutely no potash was used “over which this chymist had distilled his animal oil several times,” but potash that was first mixed with dried beef blood and heated to a red glow (see Fig. 12). This made a huge difference, chemically.
 |
| Figure 12. 1732: The discovery of Prussian blue (Dippel’s version). Original transcription of the entry in Senckenberg’s diary from August 22, 1732: Pariter wolte ein mal zu einer arbeit Dippelius das sal volatile viel haben, calcinirte tartarum v. that ihn zu dem getrockneten ochsen-blut, v. trieb. Das residuum Sal, so wohl 6 Pfund waren in Capite mortuo, ex Sale alcali tartari constans et sale sanguinis, uti & portiuncula salis volatilis fixata, wolte C. D. wegwerfen, sed Rößer quicum admonebat omnia conservare | : der alles aufhub v. immer ein Register darüber hielte : | elixivabat, et conservabat im zuckerglas v. stellte es hin, ward feucht noch. Damals hatte C. D. bey sich Lieutenant Dießbach, der Florentiner lack v. andere farben machte, der wolte ex sale tartari, florent. lack machen mit Alaun, ochsenblut p ergriff diß glas, wo Rößer so schon weg war, aufgeschrieben hatte, allein, Sal tartari. Aber er bekam loco rubri coloris, caeruleum Berolinense, machte hinter C. D. her einen accord mit den Mahlern, ihnen so v. so viel zu liefern gerieth aber auf den strumpff, da das glas auß war v. es keine Farbe mehr gab so s blau aussahe mit dem gewöhnlichen gemeinen sal tartari. Kam zu C. D. v. referirte es ihm der wuste es, sagte ihm er solle nehmen sal tartari mit sanguine bovino so werde ers bekommen, v. gerieth, also. [23] |
8.2 The Discovery of Prussian Blue According to Johann Konrad Dippel (1732)
Despite the contradiction, Stahl’s distorted version continued to be recounted, with a wide assortment of embellishments, for over 300 years. It was only a few years ago that new facts came to light from an unexpected place. The results of a research project entitled “Scientific Transcription and Online-Based Provision of the Diaries of Johann Christian Senckenberg (1707-72)” at the Goethe University in Frankfurt am Main, Germany, were made publicly accessible in 2016 [23, 20, 25].
In a years-long, arduous effort, two scientific collaborators, Dr. Vera Faßhauer and Dr. Veronika Marschall, transcribed the first 13,000 (!) pages of the handwritten diaries kept by this physician and natural scientist. He maintained his diaries scrupulously from the age of 23 onwards. The entries, written in code words and with his own abbreviations in a mixture of Latin, German, French, Greek, Hessian dialect, and occasional alchemical symbols are among the most unreadable documents in the history of the city of Frankfurt.
 |
|
Johann Christian Senckenberg |
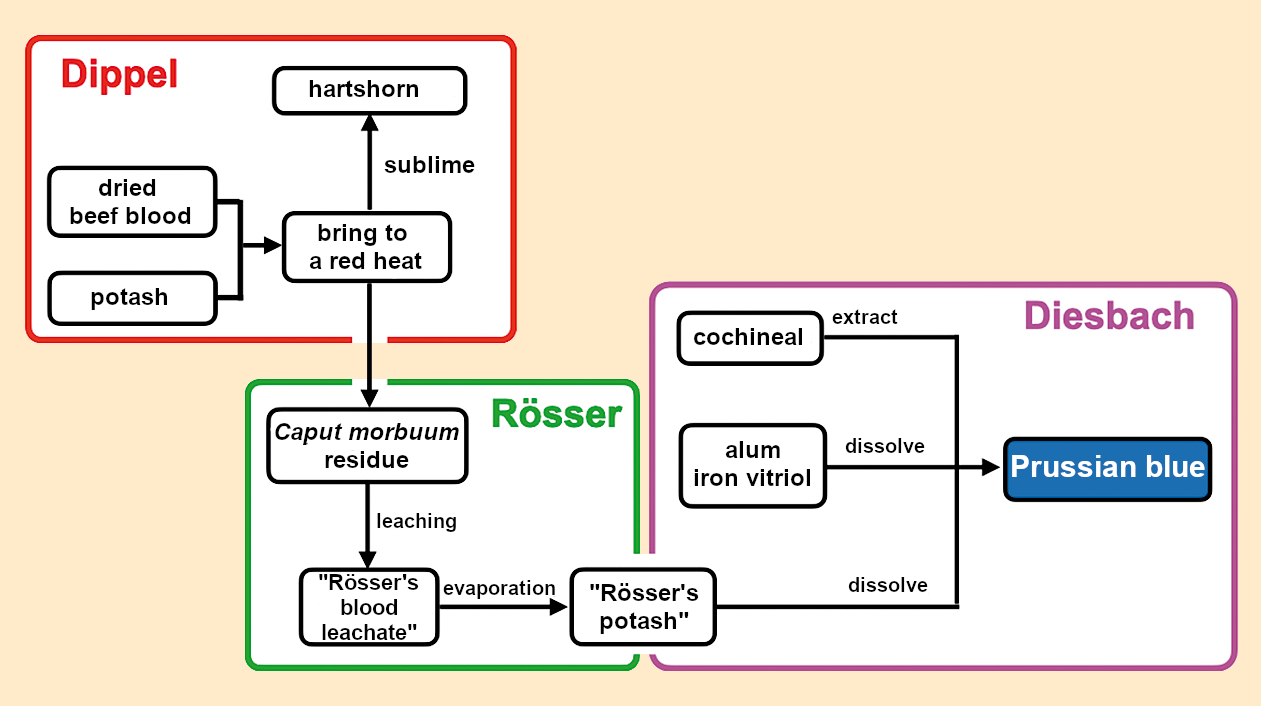
On page 406 of the second volume, Senckenberg recorded Dippel’s report about the discovery of Prussian blue on August 22, 1732 (see Fig. 12). Although Dippel was accused of “having much too high an opinion of himself,” Senckenberg’s notes show that Dippel chatted modestly about his role in the discovery of Prussian blue in this informal conversation. If we trust Dippel, and there is no reason not to, his statements provide some clarity about the behavior of the chymists involved in the discovery (see Fig. 13):
 |
|
Figure 13. First synthesis of Prussian blue according to Dippel (1732). |
At one time, Dippel and Rösser produced a large amount of hartshorn by the calcination of potash with dried beef blood. This resulted in “about 6 pounds” of residue (Caput morbuum). Dippel wanted to throw the residue away, but Rösser leached it out, boiled down the leachate, and put the resulting solid into a container on which he wrote “potash”.
Lieutenant Diesbach wanted to produce Florentine lake. Unknowingly, he used the potash from the container labeled by Rösser. This did not make a red color, but made Prussian blue instead.
Diesbach continued to produce Prussian blue behind Dippel’s back and committed to “delivering a certain amount” to his clients. Diesbach thus found himself in a predicament when the container was empty because the “ordinary, nasty potash” did not produce any Prussian blue. Diesbach had to go to Dippel and ‘”report” to him. Dippel advised him to first calcinate the potash with beef blood, “that way he would obtain it, and so he did!”
9 The Search for the “Colored Material” in Prussian Blue
His unplanned reprocessing of the calcination residue from the hartshorn production process allowed Rösser to link the completely independent recipes for hartshorn (see Fig. 1, Part 1) and Florentine lake (see Fig. 4, Part 1). Why it was that a blue pigment was suddenly obtained despite the use of red cochineal pigment remained a mystery for a long time.
9.1 The Insatiable Curiosity of the Chymists
Let us enter the world of the chymists of the time. They were certainly proud of “their” Prussian blue, but it must have been demoralizing for them that the recipe essentially fell from the sky and that no one had the slightest idea why it worked. John Brown, who verified the recipe that was anonymously submitted to the Royal Society in 1724, clearly expressed how much this bothered him:
“One would really like to know what gave the first indication that this combination of different materials could produce such a fine color, especially when one considers that blood plays the main role in this surprising color change.”
He began to undertake experiments with Prussian blue himself and was able to demonstrate that alum was unnecessary, but blood and potash were essential, and that the iron could not be replaced with any other metal. Based on this, he proposed the following “reaction mechanism”:
- The blood confers its “colored material” to the potash during the calcination reaction.
- The blood leachate obtained from the calcination residue contains potash and “colored material”.
- The blood leachate transfers the “colored material” from the potash to the iron and gives it this splendid color.
At the time, there were also completely divergent ideas. For example, French chemist Abbé François Ménon was of the opinion that the color “is nothing more than a dark blue that is caused by the natural color of the iron.” [26]
9.2 Over 100 Years of Research into Prussian blue
This reasoning, plausible at the time, inspired generations of chymists to search for the “colored material” in Prussian blue. They seized upon all chemical agents in attempts to break Prussian blue down and separate out the iron contained within. Progress was slow, however, and many clever researchers wracked their brains. Here are a few examples from over 100 years of Prussian blue research:
1725 Étienne François Geoffroy (1672 – 1731) heated Prussian blue. It gave off ammonia and a charred mass remained.
1752 Pierre-Joseph Macquer (1781 – 1784) stirred Prussian blue into a freshly prepared solution of potash. The blue color disappeared and a brown solid precipitated [19]. He believed that the iron in the Prussian blue had given its “colored material” back to the potash and then precipitated out as brown iron hydroxide.
1774 Jean Hellot (1685 – 1766) presented to the Academie Royale des Sciences: “There may be nothing more bizarre than the method for producing Prussian blue. It must be admitted that if chance hadn’t played a role, a profound theory would have been necessary to conceive of this process.”
1771 Carl Wilhelm Scheele (1742 – 1786) discovered the element nitrogen.
1774 Johann Georg Krünitz (1728 – 1796), natural scientist and lexicographer, got lost in all the confusion and under the heading “Prussian blue” in the fourth volume of his “Economic Encyclopedia” resignedly wrote, “After the chymists discovered this Prussian blue, they attempted to develop the theory of it and to explain what it is that happens in these various operations. However, because this work seems to be too far removed from the actual purpose of my work, I cannot enter further into it.”
1782 Carl Wilhelm Scheele (1742 – 1786) boiled Prussian blue with sulfuric acid and distilled the “colored material” over with water. The “colored material” proved to be a water-soluble acid that he called acidum berolinense, which later became Prussian blue acid, blue acid, and acide prussique. By adding iron(II) sulfate to the blood leachate, Scheele obtained a solution of a yellow salt that consisted of “alkali, a little siderite, and the colored material” and which became known as yellow prussiate of potash.
1806 John Dalton (1766 – 1844) introduced his hypothesis of the atom.
1807 Humphry Davy (1778 – 1829) electrolytically isolated potassium from molten potash.
1811 Joseph Louis Gay-Lussac (1778 – 1850) synthesized pure acide prussique for the first time and coined the terms cyanogen (Gr. Kyanos “blue”; gennan “to generate”) and cyanide because of its relationship to Prussian blue. In 1815, Gay-Lussac proved that the composition of “blue acid” was HCN [27]. The original details of the composition he proposed have to be corrected for the fact that it was not yet known that hydrogen and nitrogen are found as H2 and N2.
1832 Swedish chemists Jöns Berzelius (1779 – 1848) and Carl Gustav Mosander (1779 – 1858) recognized that yellow prussiate of potash is a double salt consisting of potassium cyanide and iron cyanide, and that Prussian blue is a triple salt in which some of the potassium ions in the yellow prussiate of potash are replaced by iron(III) ions.
9.3 Identification of the “Colored Material”
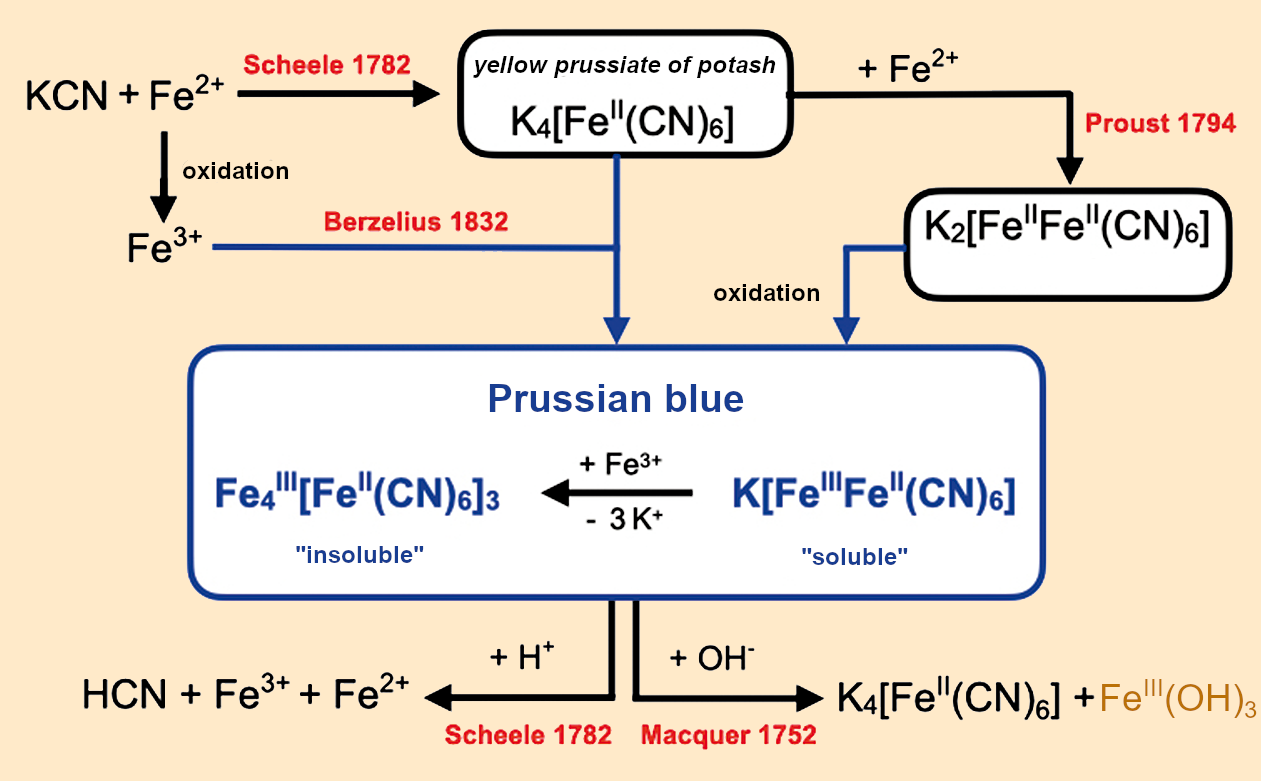
Thus, it took over a century to identify the “colored material:” It was cyanic acid (HCN), or more specifically its salt, potassium cyanide (KCN). In combination with the discovery of yellow prussiate of potash K4[Fe(CN)6], a double salt that forms from iron(II) salts and potassium cyanide in aqueous solution, these findings opened up an entirely new area of chemistry. Figure 14 shows a small part of this that involves “soluble” FeIIIK[FeII(CN)6] and “insoluble” Fe4III[FeII(CN6)3] Prussian blue.
 |
|
Figure 14. The chemistry surrounding Prussian blue. |
The solid-state structure of Prussian blue is complex, and the water and potassium content depend significantly on the conditions under which it is produced [28]. Proposed general chemical formulas include: Fe4III[FeII(CN)5]3•x K4[FeII(CN)6]•n H2O with x = 0.3–0.8 and n = 3–17 [29] or KxFeIII[FeII(CN)6][(3+x)/4] with x = 0–1 [30,31].
9.3 Where Does the Potassium Cyanide Come From?
This simple question leads to a chemical dilemma. If the “colored substance” were both a component of dried beef blood and cyanic acid or its salt potassium cyanide, blood would be highly toxic. This is clearly nonsense. However, Prussian blue is only produced if beef blood is used.
There is only one explanation for this: The “colored material” is not a component of the blood but is formed during calcination.
What Occurs Chemically in a Calcination?
Under dry heating, biological material is chemically decomposed through innumerable reaction cascades. The sequence in which the countless decomposition products are formed, the amounts produced, and the further reactions they undergo depend on the composition of the starting material and the reaction conditions, such as the temperature profile. The chemistry involved is highly complex and lies beyond the limits of our knowledge.
In the chemist’s crucible at temperatures above 850 °C, things become a little clearer, because few materials are stable under such harsh conditions. The products include mainly gases, such as water vapor, nitrogen, carbon monoxide, carbon dioxide, and ammonia, as well as the solids that remain in the crucible, like carbon and various metal salts and oxides. In carbonization, another thermally stable gas can be formed, cyanic acid or HCN (b.p. 25.7 °C).
Today we know that cyanic acid is always formed when enough energy is added to a mixture of substances containing C, H, and N [32], for example, when smoking a cigarette. With every puff, a smoker inhales a small amount of cyanic acid [33].
In contrast to dry carbonization, the production of Prussian blue involves the calcination of dried beef blood together with potash (m.p. 891 °C). The cyanic acid that is formed immediately reacts with the strongly basic potash to make potassium cyanide, which accumulates as the calcination progresses. Potassium cyanide is very stable and melts and boils without decomposition at 634 °C and 1625 °C, respectively:
2 HCN + K2CO3 → 2 KCN + H2O + CO2
Now it becomes clear: After the carbonation, the residue in the crucible contains potassium cyanide as well as carbon and potash. This potassium cyanide can only form because dried beef blood contains so much protein, which consists of up to 14 % nitrogen. Upon calcination, the nitrogen is converted first to HCN, which reacts with potash to form KCN. After leaching of the cooled residue with water and subsequent filtration, the result is the potassium cyanide-containing blood leachate. After this is evaporated, we are left with potassium cyanide-containing potash, which was termed “Rösser’s blood leachate” or “Rösser’s potash”.
Because the formation of potassium cyanide is essential for the production of Prussian blue, the version of the discovery story proposed by Stahl can be ruled out (see Chapter 8.1). Potassium cyanide would have to be formed in the distillation of the animal oil over potash, but this is not the case. Even after boiling Dippel’s oil over potash at atmospheric pressure for several hours, no cyanide can be detected.
How Grave Was the Danger to the Health of the Chymists?
Potassium cyanide and cyanic acid were unknown to the chymists of 1706, and young Rösser could have had no idea what was dissolved in the blood leachate he was working with. To estimate the danger into which Rösser could have unknowingly plunged himself, we can analyze the preparation method for Prussian blue at the time.
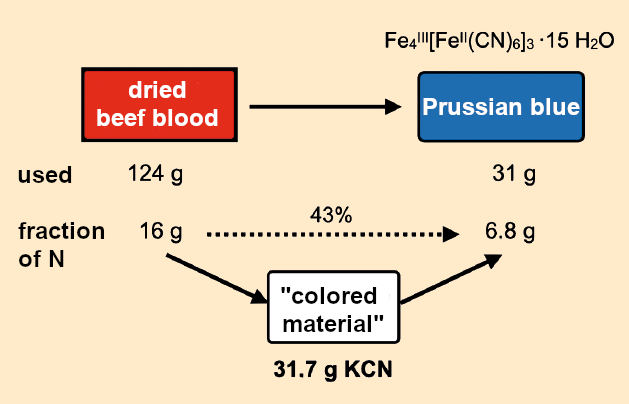
Because Dippel, Diesbach, and later Frisch never gave away any details of the amounts they used, we must resort to using John Brown’s recipe from 1724 [18]. After a two-hour calcination of 124 g dried blood and 248 g potash, he obtained a total of 31 g of Prussian blue, “or a little more.” The nitrogen balance of Brown’s reaction can be assessed as follows (see Fig. 15):
 |
|
Figure 15. Nitrogen balance in Brown’s preparation (1724). |
Because of the relatively imprecise amounts and unknown purities of the materials used and the resulting products, this nitrogen balance is only a rough estimate. It is based on the chemical formula for “insoluble” Prussian blue FeIII[FeIIIFeII(CN)6]3•15 H2O (M = 1,129 g mol–1).
- 31 g of the end product, Prussian blue, contained 6.8 g of nitrogen.
- 124 g of the dried beef blood used as a starting material contained 16 g nitrogen.
- The 6.8 g of nitrogen in the Prussian blue corresponded to 17 g of potassium cyanide.
- These 17 g of potassium cyanide were contained in “Rösser’s blood leachate”. At this point we shudder to think about young Rösser, completely cluelessly messing around with cyanide solution.
- The 17 g of potassium cyanide were produced during the calcination of 124 g dried beef blood.
- In all, 43 % of the nitrogen in the dried blood was incorporated into the Prussian blue.
This nitrogen balance indicates an impressive yield, given the brutal reaction conditions. It is not surprising that the production of potassium cyanide from potash and slaughterhouse waste (blood, hooves, claws, horns, bristles, leather, etc.) was a common industrial process in the growing cyan industry of the early 19th century [34].
References
[23] V. Faßhauer, V. Marschall, Johann Christian Senckenberg (1707 – 1772) – Tagebücher, Universitätsbibliothek J. C. Senckenberg, Frankfurt am Main, Germany.
[24] P. J. Macquer, Chymisches Woerterbuch, Erster Theil, Leipzig, 1788.
[25] H.-H. Eulner, Johann Christian Senckenbergs Tagebücher als historische Quelle, Medizinhistorisches J. 1972, 7, 233–243.
[26] F. Lankischens Erben, Auserlesene Abhandlungen der Königlichen Akademie der Wissenschaften zu Paris, Erster Theil, Leipzig, 1752.
[27] J. L. Gay-Lussac, Recherches sur l’acide prussique, Ann. Chim. 1815, 95, 136–251.
[28] A. Ludi, Berliner Blau, Chem. Unserer Zeit 1988, 22, 123–127. https://doi.org/10.1002/ciuz.19880220403
[29] A. V. Vyboishchik, M. Yu. Popov, Contemporary methods for production of Prussian blue, IOP Conf. Ser. Mat. Sci. Engin. 2020, 962, 022035. https://doi.org/10.1088/1757-899X/962/2/022035
[30] F. Shiba, U. Mameuda, S. Tatejima, Y. Okawa, Synthesis of uniform Prussian blue nanoparticles by a polyol process using a polyethylene glycol aqueous solution, RSC Adv. 2019, 9, 34589–34594. https://doi.org/10.1039/C9RA07080J
[31] F. Grandjean, L. Samain, G. J. Long, Characterization and utilization of Prussian blue and its pigments, Dalton Trans. 2016, 45, 18018–18044. https://doi.org/10.1039/C6DT03351B
[32] E. Gail, S. Gos, R. Kulzer, J. Lorösch, A. Rubo, M. Sauer, R. Kellens, J. Reddy, N. Steier, W. Hasenpusch, Cyano Compounds, Inorganic, Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. https://doi.org/10.1002/14356007.a08_159.pub3
[33] S. Streller, K. Roth, The Chemistry of Tobacco, ChemistryViews 2014. https://doi.org/10.1002/chemv.201400096
[34] H. Andreas, Mitt. Fachgruppe Gesch. Chemie 2019, 25, 47.
The article has been published in German as:
- Berliner Blau – Entdecker und Verräter,
Klaus Roth,
Chem. unserer Zeit 2021, 56, 34–49.
https://doi.org/10.1002/ciuz.202100033
and was translated by Caroll Pohl-Ferry.
Prussian Blue: Discovery and Betrayal – Part 1
Around 1700, Berlin was a colorful center of innovation – a scene that led to the discovery of the century: Prussian blue
Prussian Blue: Discovery and Betrayal – Part 2
How Johann Konrad Dippel almost ruined the Prussian blue business
Prussian Blue: Discovery and Betrayal – Part 3
Who leaked the secret recipe for Prussian blue?
Prussian Blue: Discovery and Betrayal – Part 5
Solving the case of the discovery of Prussian blue
See similar articles by Klaus Roth published on ChemistryViews.org




Congratulations to Klaus Roth! The one thing missing is to span out an arch to our contemporary knowledge about prussian blue and its analogues. And not to forget to mention the intrinsic irony which is hidden beneath the term “first man-made blue pigment of the modern era…”. There should have been plenty amounts of prussian blue and its analgues in the very early stages of the earth, as there were plenty amounts of cyanic acid, iron in both oxidation states +II and +III (yes! because of the reaction pathway from Fe(OH)2 towards Fe3O4 (… remember the Schikorr reaction, there never was a need for free Oxygen to see huge amounts of haematite building up to deposits!) and therefore to both oxidation states of iron. So, during the Hadean epoch there probably were already huge deposits of prussian blue(s) around, which intrinsically acted as RedOx buffer, explicitly as electron acceptors for ongoing processes towards emerging reproductive stages of life. Later on, whith the advent of “the great oxidation”, those iron blues most probably vanished, as they were simply oxidized and hydrolyzed to iron(III) species. Therefore, we should consider the innate capabilities of the iron blues as the primordial example for the “agency” of substances in the critical zones of the early earth (…tribute to Bruno Latour!). Consequently, any picture of the early, pre-biotic earth should be painted in white, blue red and green, with sprinkles of a golden hue (Fe2S). Prussian blue most probably was there before any kind of self-reproductive pre-biotic form on earth and the latest “civilization” did not much to discover it, but once again stumbled upon its appearance… And last, but not least, the prussian blues are already opening an avenue towards cheap, earth-abundant unlimited (and unlimitable) materials for divalent calcium batteries, for power storage and decarbonization of our economy. The blue Planet may be transformed another time, by integration of those agencies like the RedOx-moulators provided by the inorganic world.