Diols which are conformationaly favorable are specifically activated by LiCl
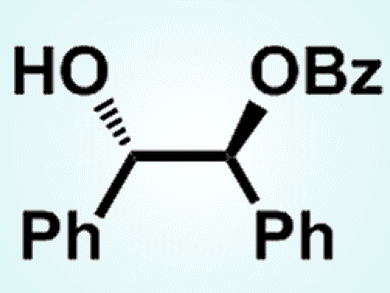
Selecting a Hydroxy Group with LiCl
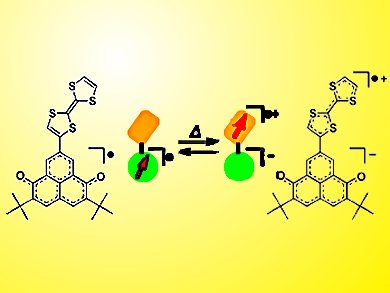
Radical Transfer
Spin-center transfer is modified by solvent and temperature in a neutral radical system
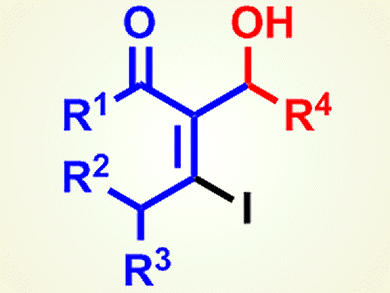
Building-Block Compounds from Iodoaldol Reactions
The iodoaldol reaction of internal alkynyl ketones gives useful oxygen-functionalized vinyl iodides
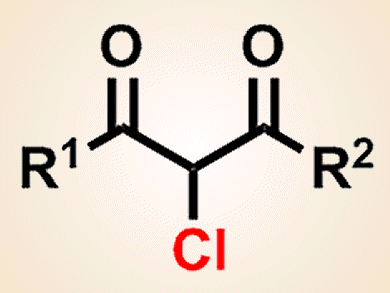
Cheaper Chlorination of Organic Compounds
Inexpensive hydrochloric acid is promising for low-cost electrochemical chlorination
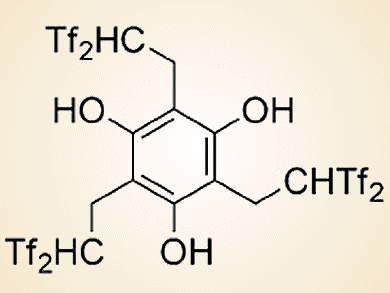
Switching Catalysts for Choosing Products
Organic bis(trifluoromethanesulfonyl)methyl-based catalysts prove versatile for selecting aldol or olefination products
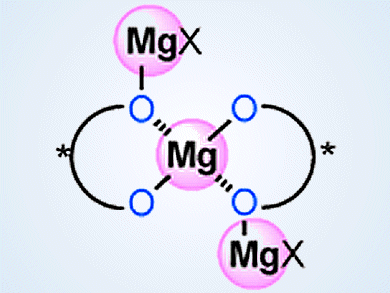
Supramolecular Catalysts from Simple Components
Magnesium/BINOL supramolecular catalysts made for phosphorus addition, Mannich-type, and hetero Diels-Alder reactions
![[4+2] = Cyclohexanones](https://www.chemistryviews.org/wp-content/uploads/legacy/common/images/thumbnails/source/1420f3c3702.gif)
[4+2] = Cyclohexanones
The 3-hydroxy group is the key to controlling cycloaddition reactions of 3-hydroxycyclobutanone
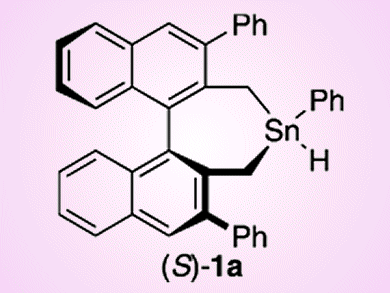
Chiral Tin Participates in Radical Cyclizations
Chiral tin hydrides generate radicals and transfer chirality in the cyclization of aldehydes
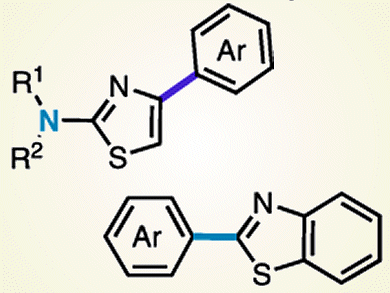
Breaking Bond
Which do you prefer to break: a C–H bond or a C–N bond?
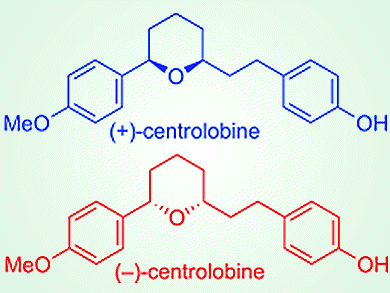
Asymmetric Esterification for Total Synthesis
Production of chiral carboxylic esters proves a useful method in the total synthesis of centrolobine