Balloons transport us into many a childhood dreamland—and come from the Hevea brasiliensis plant. The transformation of a creamy, white natural substance into a colorful balloon requires a helping hand from the gods, and only Vulcan—the Roman god of fire and a gifted chemist—can perform such a miracle. We have not yet seen through all his tricks, but we will try to follow his chemical trail.
In this part, we will look at how latex is collected, transported, and converted to natural rubber
4 Birthplace of Balloons: The Rubber Plantation
4.1 Latex Harvest
Balloon manufacture begins with the latex harvest on one of many giant rubber plantations in southeast Asia (see Fig. 9). Latex is the sap of the rubber plant (see Part 1).

Figure 9. Harvesting latex on a plantation.
Creative Commons Attribution-Share Alike 3.0 Unported
https://upload.wikimedia.org/wikipedia/commons/4/49/Latex_-_Hevea_-_Cameroun.JPG
Before the arrival of Columbus, rubber was obtained by indigenous peoples from the latex of native rubber trees, which can grow to a height of up to 30 m. Although the export of seeds was strictly prohibited to preserve the Brazilian monopoly, English biologist Henry Wickham smuggled 70,000 seeds to England in 1876, from which 2700 seedlings were grown at London Botanical Garden (Kew Garden). This was the foundation for the giant rubber plantations in the British colonies of southeast Asia. Malaysia, India, Indonesia, and Thailand dominate the global market to this day.
It remains unclear exactly how Henry Wickham smuggled the seeds out: some say it was in two stuffed crocodiles, some say a coffin, and some say it was with forged export papers. Whatever the means were, Wickham received a large amount of money for his smuggling and was later honored for the profits he earned for his home country.
The rubber harvest begins with scoring the bark of rubber trees that are at least five years old. Today, at most 180 diagonal cuts are made on one tree in a given year. After eight years the harvesters return to the first cut, where the bark has had time to completely regenerate. Small cups at the lower end of each cut collect 20–30 g of latex daily. Cultivation made it possible to increase the yield to values of over 2000 Kg/ha/a. Altogether, one third of the 15.5 million tons of rubber currently used every year is natural rubber [11]; the rest is synthetic rubber, primarily based on styrene-butadiene and butadiene.
Table 1. Composition of fresh latex.
| Component | Amount in wt% |
| total solids content | 41.5 |
| rubber | 36.0 |
| amino acids and N bases | 0.3 |
| neutral lipids | 1.0 |
| proteins | 1.6 |
| phospholipids | 0.6 |
| salts (K, P, and Mg) | 0.5 |
| water | 58.5 |
Latex is a fine dispersion of solid rubber particles (0.1 wt%), see Table 1. Each particle is encased in a phospholipid shell containing capsid proteins. The side chains of these capsid proteins, consisting of many amino acids, protrude from the surface of the particle into the surrounding water. Because their carboxyl groups are partially dissociated (i.e., have split off a proton), the rubber particles are outwardly negatively charged. It is the electrostatic repulsion between the rubber particles that keeps the latex dispersion stable.
Almost 90 % of latex is immediately processed on the plantation to make natural rubber. Acetic acid is used to lower the pH value of the latex. This protonates the carboxyl groups on the particle surface, rendering them electrically neutral. The rubber particles coagulate immediately and collect on the surface. After some time, the semisolid layer is skimmed off, rolled into disks, and hung up to dry. This is the feedstock for the wide spectrum of products made by the rubber processing industry, from wire insulation to car tires.
12 % of the latex harvest is sold as a concentrate and then processed further using the dipping process. The aim in producing thin layers by the dipping process is to induce a slower and finer coagulation so that the layers are uniform. Over half of this product is processed to make medical materials such as gloves, catheters, and tubes, and about 3 % is made into condoms and balloons.
4.2 Transportation of Latex
Dipping technology is not new: indigenous peoples produced bottles and head-coverings by spreading multiple layers of latex onto hollow, clay forms. Once the desired thickness was attained, the inner form was smashed, and the waterproof container was ready.
Balloon production is based on the same principle to this day: a form is dipped into a latex solution, dried, and then processed further. For the production of balloons in Germany, latex must be concentrated on the plantation to reduce transportation costs. It must then be treated for transportation because of its sensitivity.
Concentration
To lower the water content, the latex is centrifuged to increase the solids content to about 60 %. This correspondingly reduces the water content from 65 to 40 %.
Preservation
To inhibit coagulation during transport, the latex is treated with ammonia on the plantation. A pH value around 10 guarantees that the carboxylate groups on the protein shell encasing the particles remain deprotonated and negatively charged. The high pH value and the addition of 0.04 % ammonium laurate and 0.025 % zinc oxide/tetramethylthiuram disulfide (5) stabilizes and preserves the latex, preventing microbiological degradation.
Ammonium laurate is the ammonium salt of lauric acid, a linear, saturated, fatty acid, CH3-(CH2)10-COOH. This salt is a surfactant and stabilizes the latex particles. Its non-polar hydrocarbon residue lodges in the phospholipid layer and its negative carboxyl group protrudes outward, increasing the negative charge of the individual rubber particles.
Tetramethylthiuram disulfide (5) (see Fig. 10) is referred to by various terms: thiuram, thiram, thurad, etc. Its chemical name is bis(dimethylthiocarbamoyl) disulfide and it is a powerful fungicide and preservative.
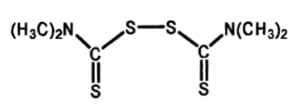
Figure 10. Tetramethylthiuram disulfide (5).
Reference
[11] Sabine Mayer, Rudolf Zentel, Andreas Greiner, Dagmar Ulbrich, Martin Vollmer, Makromolekulare Chemie 2001, Nachr. Chem. 2002, 50, 350. https://doi.org/10.1002/nadc.20020500312
The article has been published in German as:
- Die Chemie des Luftballons,
Klaus Roth,
Chem. unserer Zeit 2005, 39, 282–289.
https://doi.org/10.1002/ciuz.200590054
and was translated by Caroll Pohl-Ferry.
The Chemistry of Balloons (and Rubber) – Part 1
The balloon is certainly not the most important product based on rubber, but it may be the prettiest
The Chemistry of Balloons (and Rubber) – Part 2
The discovery of vulcanization—a happy accident or the result of hard work?
The Chemistry of Balloons (and Rubber) – Part 4
How balloons are made and why rubber products can contain harmful nitrosamines
See similar articles by Klaus Roth published in ChemistryViews