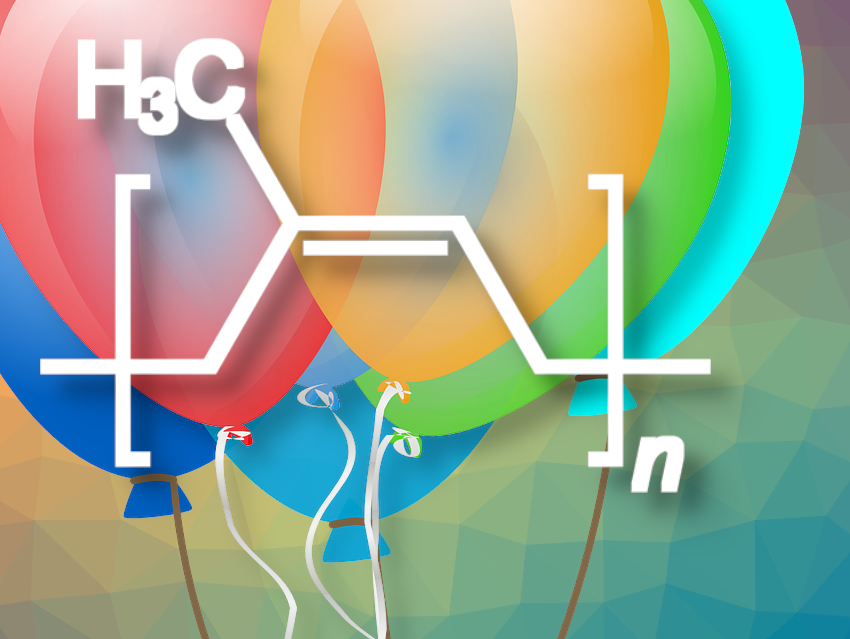Balloons transport us into many a childhood dreamland—and come from the Hevea brasiliensis plant. The transformation of a creamy, white natural substance into a colorful balloon requires a helping hand from the gods, and only Vulcan—the Roman god of fire and a gifted chemist—can perform such a miracle. In this part, we will look at how balloons are made and why rubber products can contain harmful nitrosamines.
5 The Balloon Factory
The skin of a balloon is required to meet almost impossible demands: It must be whisper-thin so that the balloon is strong and rises briskly when filled with gas. It must have a uniform thickness so that the balloon has no weak spots to cause it to pop prematurely. It must be elastic so that the balloon can be inflated multiple times. It must be storable, and the balloon must withstand multiple inflations. The balloon must be easy to inflate. The skin must be as gas-tight as possible so that the balloon stays aloft for a long time. It must not have a disgusting taste and must under no circumstances emit any compounds that are health hazards. Balloons must be available in all colors, the color must be evenly distributed, and finally, balloons need to be as cheap as possible. It is with great respect that we acknowledge that serious balloon manufacturers meet all these requirements. Here, we continue to follow the production process.
5.1 Processing the Latex Concentrate
After delivery (see Part 3) sulfur, catalysts, activators, and preservative substances are added to the latex concentrate (see Part 2). The precise formulations used are well-guarded company secrets—and for good reason, because though latex concentrate is the material basis, the quality of a balloon is primarily determined by this formulation and additional clever manufacturing tricks.
The completed mixture is pre-vulcanized through gentle heating over several hours. This leads to some degree of crosslinking between the isoprene chains via sulfur bridges (see Part 2). The desired organic pigments are then mixed into the pre-vulcanized latex concentrate. Because balloons “come into contact with the oral mucus membranes,” food and consumer goods laws only allow the use of certain approved pigments. Of these, the consumer favorite is unquestionably a bright red.
Modern balloon production is fully automated (see Fig. 11), and production numbers are remarkable: The only balloon factory operating in Germany, the Everts company in Datteln, produces up to one million (!) balloons a day.
 |
|
Figure 11. Balloon machine, approximately 30 m long, can produce many hundreds of thousands of balloons daily. |
5.2 Cleaning the Molds
The blank molds used in the dipping process (see Part 3) are first carefully cleaned in a sequence of cleaning baths.
 |
|
Figure 12. Balloon molds. |
5.3 Coagulant Bath
The goal of this production step is to apply a thin coat of coagulant to the molds. This substance causes the rubber particles to coagulate as finely and evenly as possible as the molds are dipped into the latex mixture. Calcium salts have proven successful in this role, because the doubly positive ions cancel the negative charges of the carboxylate groups on the surface of the rubber particles, removing the electrostatic repulsion between the rubber particles.
5.4 Dipping in Latex and Drying
Once covered in a uniform coat of coagulant, the molds are dipped into the latex mixture and then dried. The latex mixture contains all the additives, catalysts, activators, preservatives, and pigment (see Part 2).
This is where one of the advantages of pre-vulcanization comes into play: As the latex dries, the ends of the unpolar polyisoprene chains protrude out of the membrane shells of the pre-vulcanized latex particles. These ends then begin to loosely bind individual rubber particles together like Velcro.
At this point, everything has come together on the mold: the substances for coagulation of the latex particles, the latex particles themselves, and the substances for vulcanization.
5.5 The Lip
Balloons are only easy to inflate if the mouthpiece has a suitable end for the lips to hold (see Fig. 13). To form this lip, the upper edge of the latex mixture is loosened from the mold and folded over on itself by a roller. Because the latex layer has not yet been fully vulcanized, the individual layers adhere to each other, forming a compact ring after vulcanization.
 |
|
Figure 13. Balloons with lips. |
5.6 Post-Vulcanization
After another wash to remove coagulant residues, the final vulcanization takes place. This is where the advantages of two-step vulcanization become apparent: Pre-vulcanization takes place at slightly elevated temperatures, and post-vulcanization takes place at less than 120 °C. This low thermal strain is what allows for the use of the organic pigments that give us balloons in so many beautiful colors (see Fig. 14).
 |
|
Figure 14. Balloon molds after dipping in latex mixtures with different pigments. |
The low thermal strain during pre- and post-vulcanization allows the long isoprene chains to remain intact, which gives the balloon skin its high tensile strength.
Once the pre-vulcanized latex coat has been dried, the ends of the polyisoprene chains protrude from the membrane shell of the rubber particles. As a result, many sulfur bridges are formed between the ends of the chains of polyisoprene molecules on different rubber particles in the post-vulcanization process. This is what gives balloons their outstanding elasticity.
5.7 Removal
Once they have cooled, the balloons are washed in hot water and soapy water before being mechanically removed from the molds with rollers. After careful quality control, the finished balloons are packaged and ready for shipment.
6 The Pacifier Warning
Because balloons go into the mouth, manufacturers must comply with a large number of legal regulations. Without going into the logic of regulations and their harmonization across Europe, balloons in Germany must be sold with the following warnings, among others: Not suitable for children under 3 years of age! Warning! Children under 8 years of age can choke on uninflated or popped balloons! Parental supervision is required! Keep uninflated balloons out of the reach of children! Popped balloons should be removed immediately! Made from natural rubber. According to §30 of the Foodstuff and Consumer Goods Law (LMBG), balloons may not emit any hazardous substances.
6.1 Nitrosamines in Pacifiers, Balloons, and Condoms
Because elevated concentrations of carcinogenic nitrosamines were found in pacifiers during the “pacifier panic” several years ago, the Federal Institute of Risk Assessment in Berlin (BfR) (formerly the Federal Institute for Health Consumer Protection and Veterinary Medicine, formerly the Federal Health Department) established a mandatory limit of 10 µg of nitrosamine per kg of pacifier [12].
The Institute reasoned as follows: In the worst case, an infant could suck out the entire nitrosamine content of a 10 g pacifier, resulting in the ingestion of, at most, 0.1 µg nitrosamine.
With a balloon, in the worst case, the entire 10 cm2 neck can have all the nitrosamine sucked out of it. If at most 0.1 µg of nitrosamine may be ingested, and 1 kg of balloon material has a surface area of about 400 dm2, the balloon material may contain no more than 400 µg/kg of nitrosamine (BfR 26.3.2006) [13]. However, a BfR study showed that balloons do not get sucked on as intensively or for as long as pacifiers.
The “Pacifier Panic” was repeated in 2004—again with corresponding press coverage—with another everyday item closely related to the balloon: the condom [14]. Chocolate-flavored condoms were found to have an especially high nitrosamine content, most of which could be attributed to the cocoa roasting process. Assuming adherence to the BfR regulation value of at most 400 µg of nitrosamine per kg of balloon material, a 1.5 g condom made from this material would contain at most 0.6 µg of nitrosamine. How or by whom the entire (!) nitrosamine content of a condom would be ingested I will leave to the imagination of the reader.
Before the possible carcinogenic effect of blowing up a balloon takes your breath away, you should know that an adult consumes 0.2–0.3 µg of nitrosamine with their daily meals.
6.2 Where Do the Nitrosamines in Balloons Come from?
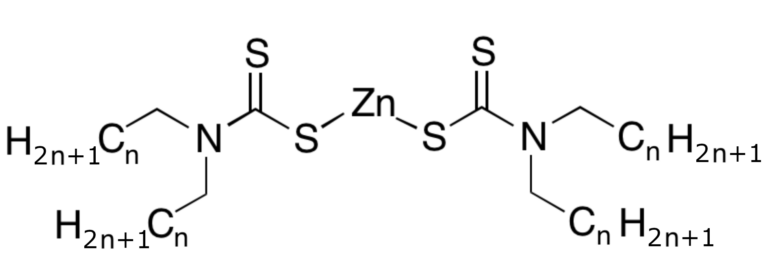
Many catalysts, like tetramethylthiuram disulfide (5) (see Fig. 10) and the preservatives added at the plantation, such as zinc N,N-dialkyldithiocarbamate (3) (see Fig. 7), degrade over time and during processing to form dimethylamine. Dimethylamine reacts with nitrogen oxides (NO and NO2) in the atmosphere to form carcinogenic dimethylnitrosamines ON–N(CH3)2.
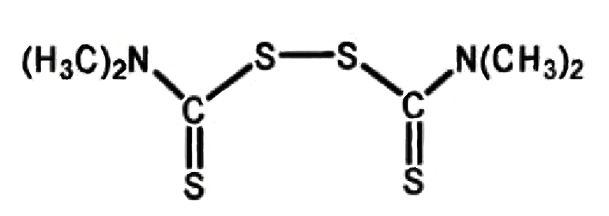 |
|
Figure 10 (from Part 3). Tetramethylthiuram disulfide (5). |
 |
|
Figure 7 (from Part 2). Zinc N,N-dialkyldithiocarbamate (3). |
However, because balloons cannot be manufactured at all without catalysts and latex preservatives, alternatives that do not result in the formation of carcinogenic nitrosamines are being sought. In contrast to dimethylnitrosamine, dibenzylnitrosamine is not carcinogenic. The use of tetrabenzylthiuram disulfide and zinc N,N-dibenzyldithiocarbamate, both based on dibenzylamine, is currently being researched.
7 Conclusion
Rubber, a sticky natural product discovered by indigenous Brazilians and marveled at by Columbus, first gained practical significance once Goodyear discovered vulcanization.
Balloons are certainly not the most important product made from rubber, but they may be the most beautiful. The industriousness and ingenuity of many generations of scientists and engineers is reflected in their low weight, beautiful color(s), lack of damaging health effects, and, most strikingly, their unbelievable elasticity.
When we are at a children’s birthday party and raise a balloon to our lips to inflate it by stretching some polyisoprene chains with the power of our lungs, we can be proud of this wonderful product. We should tell our children why this makes us proud—and enjoy the moment.
Acknowledgments
I thank Dr. E. Vaupel of the German Museum in Munich, Dr. K.-H Hellwich of the Beilstein Institute in Frankfurt, Germany, and especially Dr. Rainer Hotzelmann and Katrin Gille of Fa. Everts Balloons in Datteln, Germany, for their assistance in researching this article.
Dr. Hotzelmann’s presentation during an advanced training course of the German Chemical Society was the inspiration for this article. I thank Carina and Dr. Hubertus Pohris, Marburg, Germany, and the company Dutch Dipping Technologies of Almelo, The Netherlands, for their help and sharing of photographic materials.
References
[12] Stellungnahme des BgVV vom 11.4.2002, Risikobewertung von N-Nitrosaminen in Luftballons, www.bfr.bund.de. (accessed September 5, 2023)
[13] Ergänzende Stellungnahme des BfR vom 26. März 2004, Bewertung von Nitrosaminen in Luftballons, www.bfr.bund.de. (accessed September 5, 2023)
[14] Krebserregende N-Nitrosamine in Kondomen, Chemisches und Veterinäruntersuchungsamt Stuttgart (CVUA) 28.05.2004, cvuas.untersuchungsämter-bw.de. (accessed September 5, 2023)
The article has been published in German as:
- Die Chemie des Luftballons,
Klaus Roth,
Chem. unserer Zeit 2005, 39, 282–289.
https://doi.org/10.1002/ciuz.200590054
and was translated by Caroll Pohl-Ferry.
The Chemistry of Balloons (and Rubber) – Part 1
The balloon is certainly not the most important product based on rubber, but it may be the prettiest
The Chemistry of Balloons (and Rubber) – Part 2
The discovery of vulcanization—a happy accident or the result of hard work?
The Chemistry of Balloons (and Rubber) – Part 3
How latex is collected, transported, and converted to natural rubber
See similar articles by Klaus Roth published on ChemistryViews.org