How can a tiny molecule like ethanol be at the root of so much human misery?
Here we propose to get to the bottom of the chemical consequences of a night of celebrating to excess.
Many of us know from painful experience how the over-enjoyment of alcohol can disagree with our systems. Nevertheless, the tendency persists, over and over again, to suppress this simple bit of wisdom. The typical symptoms: after a short period of lifted inhibitions, accompanied by increasingly childish tomfoolery, usually serious problems with speech follow. Continuing to imbibe further leads to confusion and loss of orientation, as well as an inability to move the extremities in a coordinated fashion. The state of complete inebriation produces total helplessness from a fully impaired sense of equilibrium.
It‘s true that all the alcohol consumed will be completely metabolized within 8–12 hours, but the physical effects last longer. There arises what is colloquially referred to as a “hangover”, or “veisalgia” in medical terminology. The latter is in turn a word derived from the Norwegian “kveis”, for indisposition brought on by intemperance, and the Greek “algia” for pain. Typical symptoms include nausea, vomiting, equilibrium problems, general weakness, lack of appetite, dry mouth, etc.
Given that the ethanol culprit has already been metabolized by the time the first symptoms of a hangover appear, the question naturally arises: What is it that actually tortures us to the point that we may well feel closer to death than to life? Let’s look for chemical traces by tracking the course of an ethanol molecule from the first swig to the bitter end.
1. From the First Gulp to Inebriation
Organs Flooded with Ethanol
For a cold sober analysis, we reduce indulgence in beer, wine, or champagne to the disdainful oral intake of a dilute solution of ethanol. Once swallowed, ca. 10–20 % of the alcohol is absorbed already in the stomach, with the remainder being processed in the duodenum or the small intestine. A rough top-of-the-head calculation elucidates what it is we are asking of our bodies by indulging in a convivial evening.
In drinking one bottle of red wine we consume roughly 80 g of ethanol. Compared with other drugs and medicaments this is truly an enormous amount. Due to its high water and fat solubility, ethanol is thus able to penetrate all cell membranes, and over the course of a drunken festivity, every organ in the body is litera lly flooded with dilute ethanol.
lly flooded with dilute ethanol.
A bacchanalian evening begins with the pleasurable part. Moderate indulgence in alcohol permits a lax joviality to arise, perceived as an expression of zest for life. Everyday cares are forgotten for the moment. Most people, when a bit drunk, are also sure that they possess enhanced physical and mental abilities. But it’s all a delusion! Objective measurements show precisely the opposite: ethanol is not a stimulant, but acts instead as a sedative. The seeming euphoriant powers of ethanol are a function exclusively of its disinhibiting effect. In other words, upon becoming a bit drunk we think ourselves capable of things we would never dream of undertaking when sober.
Blood-alcohol Concentrations Between 0.3 to 5.0 ‰
As with all substances displaying sedative and narcotic effects, with increasing intake the subject passes through multiple stages. First, disturbances in gait are observed above a blood-alcohol concentration of about 0.3 ‰ (per mill), as well as diminished concentration and a certain amount of tunnel vision.
Above 0.5 ‰ there is an onset of mental relaxation, together with a tipsy sense of wellbeing. Individual perceptions vary considerably, however, determined in part by the momentary mental attitude: euphoria in anticipation of a positive experience, or relaxation with weariness and a willingness to doze off.
The classic signs of drunkenness, such as problems with speech and significantly retarded reactions, commence with a blood-alcohol concentration of 1 ‰.
Further intake of alcohol dulls the higher nerve centers, resulting in serious disturbance of musculature coordination, and the progressive decline in inhibitions may lead to miscalculations and overestimations, sometimes with dramatic consequences. For example, the risk to an automobile driver of experiencing an accident increases 25-fold when the blood-alcohol level reaches 1.5 ‰! Above 2.5 ‰, with breathing still intact, a deep state of unconsciousness may develop, but above 4 ‰ there is a real risk of respiratory arrest, and levels in excess of 5 ‰ are typically fatal.
Slow excessive drinking results in a coma; at some point the tippler simply keels over. Actually, he or she is lucky in this case, since it prevents consumption of the relatively small additional amounts of alcohol that would result in respiratory failure. Even so, mortal danger remains, since most alcohol deaths are a result of suffocation from one’s own vomit. A person who has passed out from alcohol should, therefore, always be placed in a lateral, recumbent position.
Lethal alcohol poisoning is often a result of “binge drinking”, in which, for example, an entire bottle of hard liquor is rapidly downed, perhaps as part of a contest. If no guardian angel is present to swiftly induce vomiting, the blood alcohol level can rise to a lethal value within half an hour, resulting in death from respiratory paralysis.
The numerical values quoted above would rise in the case of an alcoholic, due to the development of increased tolerance by the central nervous system. In fact, in 2001 there was a case recorded in Karlsruhe, Germany, of a 35-year old man being admitted to the municipal clinic with a measured blood-alcohol level of 5.8 ‰ — and he survived, thanks solely to the wonders of modern intensive-care medicine.
GABAA-Receptor
In the state of inebriation, ethanol makes a complete shambles of the entire system of communication among nerve cells, although the details remain largely a mystery. The first ethanol-sensitive GABAA-receptor (GABA = gamma-aminobutyric acid) was identified in 2006, in the cell membranes of nerve cells. A significant attenuation of neuron activity was observed upon its binding with ethanol [1, 2], which would explain ethanol’s sedative effect. Other sedatives, such as barbiturates or benzodiazepines (e.g., Valium), were found to bind also to the same GABAA-receptor.
2. Gradual Sobering-Up
As already noted, ethanol is absorbed in the stomach, the duodenum, and the small intestine. After absorption, the blood-alcohol concentration rises to a maximum in ca. 40 minutes (see Fig. 1), with 2–4 % of the absorbed ethanol eliminated unchanged through respiration, or by way of the kidneys.
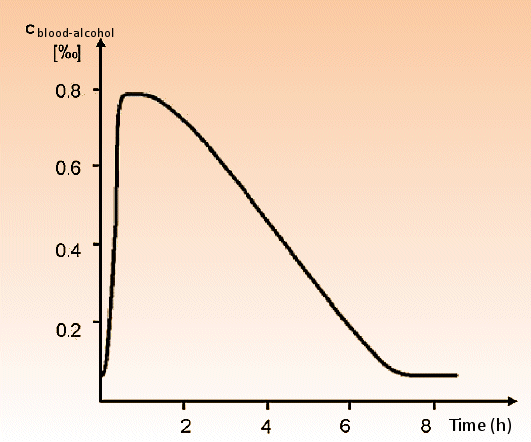
Figure 1. Blood-alcohol concentration over the course of a “celebratory evening”.
Ethanol absorption can be retarded by a full gastro-intestinal tract, but it is not diminished overall. Absorption occurs especially rapidly from beverages that are sweet, warm (e.g., grog), or carbonated (e.g., champagne). Since alcohol concentration in exhaled air is proportional to that in the bloodstream, simple breath tests make it possible to establish reliable blood-alcohol values.
Given that the most cherished aspects of intoxication are a consequence of the interaction of ethanol with the central nervous system, the metabolism of the consumed ethanol, i.e., detoxification of the body, is accomplished exclusively by the liver. Chronic mistreatment of the liver can lead to its pathological change, so it is no wonder that such changes are commonly observed with alcoholics, who typically consume massive amounts of ethanol.
Widmark Formula
A maximal blood-alcohol concentration can be obtained with the Widmark Formula: c = A / (r • W), where c is the blood-alcohol concentration in ‰, A is the amount of alcohol consumed in g, W is the weight of the person in question in kg, and r is the distribution factor in the body, with r = 0.7 for men and 0.6 for women. This distribution factor reflects the differing water content in the bodies of men and women.
For example:
A liter of beer (alcohol content 4 volume-%) represents 32 g of alcohol (density 0.8). A man weighing 70 kg (155 lb.) drinking a liter of beer thus attains a blood-alcohol content of 0.65 ‰, whereas a woman weighing 55 kg (130 lb.) would, under the same conditions, register 0.97 ‰.
After reaching a maximum, the value for both sexes decreases linearly by 0.1 to 0.2 ‰ per hour. The cause of this linear behavior is the special kinetics associated with the enzyme-catalyzed decomposition reaction. The fundamental kinetic basis (the Michaelis-Menten equation) is dealt with in every biochemistry textbook.
Individual rates of ethanol metabolism are a function of amounts and versions of alcohol dehydrogenase present in the liver cells. The actual rate of metabolism is not subject to acceleration either by drugs or by behavior (e.g., athletic activity).
► Read more on chemistry of a hangover in part 2
References
[1] M. Wallner et al., Proc. Natl. Acad. Sci. USA 2006, 103, 8540. DOI: 10.1073/pnas.0600194103
[2] H. J. Hanchar et al., Proc. Natl. Acad. Sci. USA 2006, 103, 8546. DOI: 10.1073/pnas.0509903103
The article has been published in German in:
and was translated by W. E. Russey.
Other articles by Klaus Roth published by ChemistryViews magazine:
- In Espresso — A Three-Step Preparation
Klaus Roth proves that no culinary masterpiece can be achieved without a basic knowledge of chemistry
DOI: 10.1002/chemv.201000003 - In Chocolate — The Noblest Polymorphism
Klaus Roth proves only chemistry is able to produce such a celestial pleasure
DOI: 10.1002/chemv.201000021 - In Sparkling Wine, Champagne & Co
Klaus Roth shows that only chemistry can be this tingling
DOI: 10.1002/chemv.201000047 - In The Chemist’s Fear of the Fugu
Klaus Roth shows the chemist’s fear of the fugu or pufferfish extends as far as the distinctive and intriguing poision it carries
DOI: 10.1002/chemv.201000104