Asparagus is highly prized in culinary circles. However, enjoying asparagus has an unpleasant side-effect that sets it apart from other vegetables, but is seldom talked about: It makes urine stink. This time, we’ll look at why asparagus produces odorous compounds.
3. Seeking the Source of the Smell
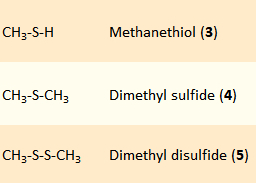 In the attempt to trace the sulfur-containing odorous substances 3–5 (pictured on the right) in urine back to their chemical source, the complexity of the biochemical processes involved—from the growth of asparagus in the field to the excretion of urine—quickly becomes evident. With some humility, we have limited our curiosity to the sulfur atoms in 3–5 and will attempt to retrace their chemical fates. Although asparagus has been known and eaten since the time of the ancient Greeks, reports of stinking urine only date back to the middle of the 17th century, a time when sulfur and sulfates were first being specifically used as fertilizers [9].
In the attempt to trace the sulfur-containing odorous substances 3–5 (pictured on the right) in urine back to their chemical source, the complexity of the biochemical processes involved—from the growth of asparagus in the field to the excretion of urine—quickly becomes evident. With some humility, we have limited our curiosity to the sulfur atoms in 3–5 and will attempt to retrace their chemical fates. Although asparagus has been known and eaten since the time of the ancient Greeks, reports of stinking urine only date back to the middle of the 17th century, a time when sulfur and sulfates were first being specifically used as fertilizers [9].
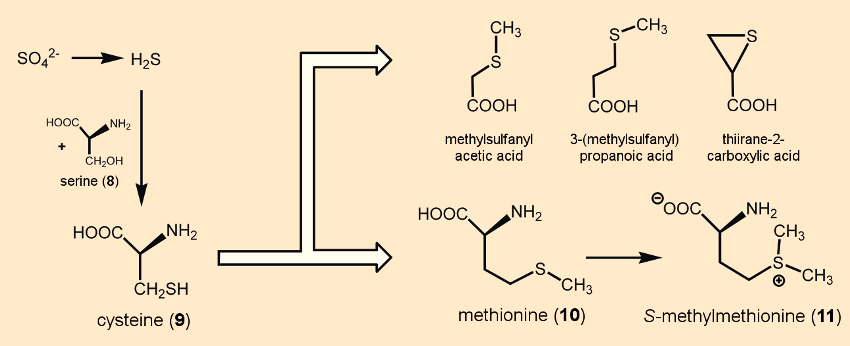
All plants take up the sulfur they require from the soil in the form of sulfates. After reduction to H2S, a reaction with serine (8) produces cysteine (9), by way of which the sulfur gets brought into the plant’s metabolism [10] (see Fig. 4). Cysteine (9) and methionine (10) form the basis of a molecular network of sulfur compounds [11].
 |
|
Figure 4. Some sulfur-containing components in raw vegetables. |
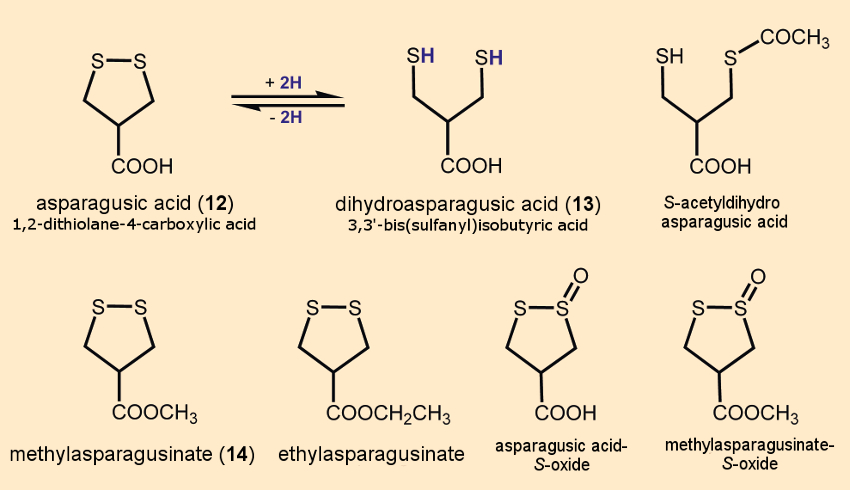
Chemically, asparagus is something special because it is the only vegetable that can synthesize asparagusic acid (12) and its derivatives [12] (see Fig. 5). Because no other vegetable results in such a strong odor in urine, we can assume that asparagusic acid lies behind the odor of asparagus urine. We will, thus, take a closer look at this acid.
 |
|
Figure 5. Asparagusic acid and some of its derivatives. |
4. Asparagusic Acid
In 1948, Eugene Jansen isolated a compound from asparagus that he knew had a disulfide bond, but he couldn’t characterize it further. After reduction with sodium in liquid ammonia, it produced 3,3′-dithio-isobutyric acid (13) [13]. A successful laboratory synthesis of this compound was carried out in 1956, and it could further be oxidized with oxygen to form the cyclic 1,2-dithiolane-4-carboxylic acid (12) [14]. It wasn’t until 1972 that 12 and 13 were recognized as natural compounds from asparagus and the carboxylic acid was given the common name, asparagusic acid [15].
Of course, asparagus doesn’t just produce asparagusic acid in order to annoy or please us; it is using its synthetic creativity in the fight for survival. Together with other plant hormones, asparagusic acid accelerates the extremely fast underground growth of the shoot [16], while also inhibiting the growth of other plants in the area. Concentrations of asparagusic acid and other plant hormones such as indole-3-acetic acid and abscisic acid are particularly high in asparagus tips. In addition, asparagusic acid is effective against various predators, so it is particularly concentrated in the delicate tips of the shoots [17].
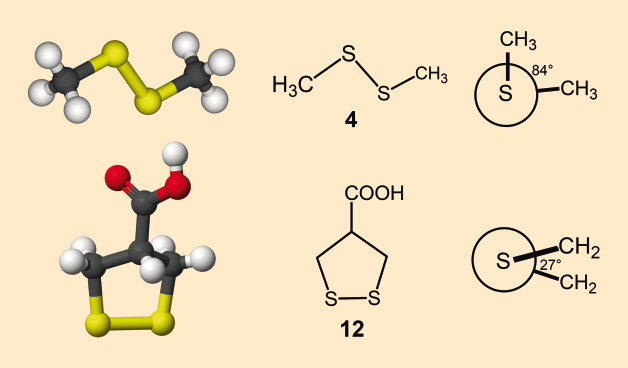
In addition to its biosynthesis [18], the chemistry of asparagusic acid is also full of surprises. The 1,2-dithiolane five-membered ring system is not flat and is sterically strained. Whereas the size of the sulfur atoms causes the R-S-S-R’ dihedral angle in open-chain disulfides to be almost 90°, it is only 27° in asparagusic acid [19] (see Fig. 6). This leads to steric hindrance of the two CH2 groups in the five-membered ring, which makes asparagusic acid more reactive.
 |
|
Figure 6. Top: The open-chain disulfide 4 has a C-S-S bond angle of 90° and a dihedral C-S-S-C angle of 84°. Below: In contrast, the dihedral angle around the disulfide bond in the five-membered ring is reduced to 27°. |
References
[9] S. C. Mitchell, Food idiosyncrasies: beetroot and asparagus, Drug Metab. Dispos. 2001, 29, 539–543.
[10] H.-W. Heldt, B. Piechulla, Pflanzenbiochemie (in German), Spektrum Akademischer Verlag, Heidelberg, Germany, 2008. ISBN: 9783827419613
[11] C. Dawid, T. Hofmann, Identification of Sensory-Active Phytochemicals in Asparagus (Asparagus officinalis L.), J. Agric. Food. Chem. 2012, 60, 11877–11888. https://doi.org/10.1021/jf3040868
[12] R. J. Parry et al., Biosynthesis of sulfur compounds. Investigations of the biosynthesis of asparagusic acid, J. Am. Chem. Soc. 1985, 107, 2512–2521. https://doi.org/10.1021/ja00294a051
[13] E. F. Jansen, The isolation and identification of 2,2′-dithiolisobutyric acid from asparagus, J. Biol. Chem. 1948, 176, 657–664.
[14] L. Schotte, H. Ström, The Preparation of 1,2-Dithiolane-4-carboxylic Acid, Acta Chem. Scand. 1956, 10, 687–688. https://doi.org/10.3891/acta.chem.scand.10-0687
[15] H. Yanagawa et al., Asparagusic acid, dihydroasparagusic acid and S-acetyldihydroasparagusic acid, a new plant growth inhibitors in etiolated young asparagus officinalis, Tetrahedron Lett. 1972, 2549–2552. https://doi.org/10.1016/S0040-4039(01)84871-1
[16] K. Kojima, N. Sakurai, IAA Distribution in Etiolated Spears of Asparagus, HortScience 1994, 29, 822. https://doi.org/10.21273/HORTSCI.29.7.822
[17] S. C. Mitchell, R. H. Waring, Asparagusic acid, Phytochemistry 2014, 97, 5–10. https://doi.org/10.1016/j.phytochem.2013.09.014
[18] R. J. Parry, Biosynthesis of some sulfur-containing natural products investigations of the mechanism of carbon-sulfur bond formation, Tetrahedron 1983, 39, 1215–1238. https://doi.org/10.1016/S0040-4020(01)91887-3
[19] O. Foss, O. Tjomsland, The Crystal and Molecular Structure of 1,2-Dithiolane-4-Carboxylic Acid, Acta Chim. Scand. 1958, 12, 1810–1818. https://doi.org/10.3891/acta.chem.scand.12-1810
The article has been published in German as:
- Vom Hochgenuss zum Gestank,
Sabine Streller, Klaus Roth,
Chem. unserer Zeit 2018, 52, 112–119.
https://doi.org/10.1002/ciuz.201800853
and was translated by Caroll Pohl-Ferry.
The Smell of Asparagus Urine – Part 1
What substances cause the characteristic smell of asparagus pee?
The Smell of Asparagus Urine – Part 2
Why does asparagus produce odorous compounds?
The Smell of Asparagus Urine – Part 3
How does asparus change when it is cooked?
The Smell of Asparagus Urine – Part 4
Does everybody’s urine smell after eating asparagus?
See similar articles by Klaus Roth published on ChemistryViews.org


