The Isenheim altar is counted among the most beautiful altarpieces in all of Western art (Fig. 1). Most especially moving is a cripple covered with boils and apparently beset with unspeakable pain. These are consequences of “St. Anthony’s Fire”, a condition that occurred in epidemic proportions, and led to the deaths of countless victims. It was a matter of centuries before “St. Anthony’s Fire” was finally recognized as the result of poisoning through the ergot fungus, thriving on the rye that served widely as a basis for bread.
In this last part, we look at how the isolation and structural determination of the potent ergot alkaloids allows us to enjoy our daily bread without harm.
Substances Present in Ergot
The spectacular pharmacological activity of ergot has long tempted pharmacists, chemists, and pharmacologists to isolate its components, and to both determine their structures and devise ways to synthesize them. In retrospect, ergot has turned out to be a chemical treasure trove [18]. In addition to high-potency alkaloids, many other biologically active compounds were isolated for the first time from this source, materials that today one would never associate with ergot:
- histamine, which is instrumental in, among other things, allergic reactions and the body’s defenses against foreign substances;
- acetylcholine, an important neurotransmitter that passes activity along from nerve cell to nerve cell; and
- vitamin D2, which forms in the human body in the upper layer of the skin as a result of a light-induced rearrangement of ergosterol [19]. Only the name of the provitamin is reminiscent of the latter’s occurrence in ergot.
Charles Tanret isolated the first crystalline alkaloid from ergot in 1875. Designated ergotinin, this later turned out to be a mixture of substances. Arthur Stoll was the first in 1918 to isolate from this source a pure component, namely ergotamine [20], to be followed by ergometrin, ergocristin, ergocryptin, and ergocornin [21, 22]. Lysergic acid provides the basic framework for all these alkaloids; its structure was clarified in 1935 [23–25] (see Fig. 7).
.gif)
Figure 7. The alkaloids of ergot.
The ergot alkaloids belong to the large category of indole alkaloids, their definitive structural element being lysergic acid. Ergot alkaloids are either simple amides of lysergic acid or else compounds in which tricyclic tripeptides are similarly attached to lysergic acid via an amide linkage. A number of other physiologically active agents are semi-synthetic derivatives of the natural ergot alkaloids. The overall alkaloid content of ergot, as well as the relative amounts of individual alkaloids, varies with place of origin and crop year. Typical relative analytical values for Canadian ergot compounds are: ergocristin (31 %), ergotamine (17 %), ergocryptin (5.3 %), ergometrin (5 %), ergosin (4.2 %), and ergocornin (4 %).
The first total synthesis of lysergic acid was reported in 1954 by Edmund Kornfeld and R. B. Woodward [26]. Building on this approach, Albert Hofmann in 1961 accomplished a total synthesis of ergotamine by attaching the tripeptide fragment to lysergic acid [27, 28]. With respect to biosynthesis, the fungus joins the amino acid tryptophan with a key building block for terpenes and steroids: isopentenyl diphosphate (see Fig. 8). The lysergic acid that results after several synthetic steps is in turn coupled through its carboxyl group in the 9-position with either a simple amine, or else a tripeptide derived from three amino acids, to give the various ergot alkaloids [29].
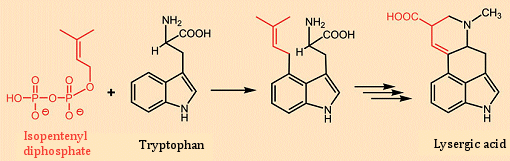
Figure 8. Biosynthesis of lysergic acid.
Lysergic acid and its derivatives are unstable in the presence of acid, as shown in Figure 9 through the example of ergotamine. Ergotamine is transformed via two proton-catalyzed rearrangements occurring in parallel to ergotaminine and the stereoisomer designated by Hofmann as an aci-compound [30], whereby the configuration change at C-8 leads to ergotaminine and that at C-2′ of the peptide unit to aci-ergotamine.
Since essentially only ergotamine is biologically active, occurrence of these isomerizations results in a decrease in ergot toxicity during storage and processing (boiling, baking). Decrease in the material’s toxicity with time is a consequence as well of temperature- and acid-sensitivity of the alkaloids, since the 9,10-double bond readily undergoes addition of water, rendering it subject to further oxidation, and the peptide portion can easily be cleaved hydrolytically.
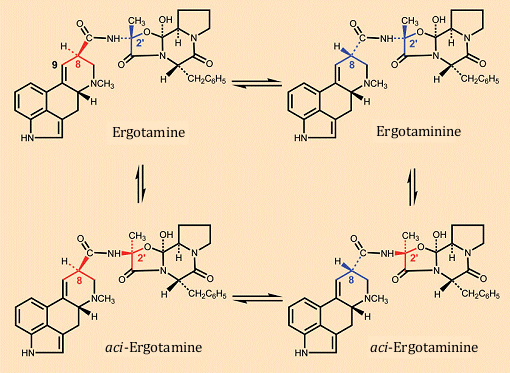
Figure 9. Acid-catalyzed rearrangement of ergotamine.
Pigments in Ergot
Ergot also contains pigments. These are associated with two major classes of compounds: the red anthraquinone pigments endocrocin and clavorugin, and a group of yellow xanthone pigments, the ergochromes (see Fig.10). Elucidation of their structures and also their biosyntheses is due especially to the work of B. Franck [31], who showed that the initially biosynthesized anthraquinones are transformed into the yellow xanthone derivatives.
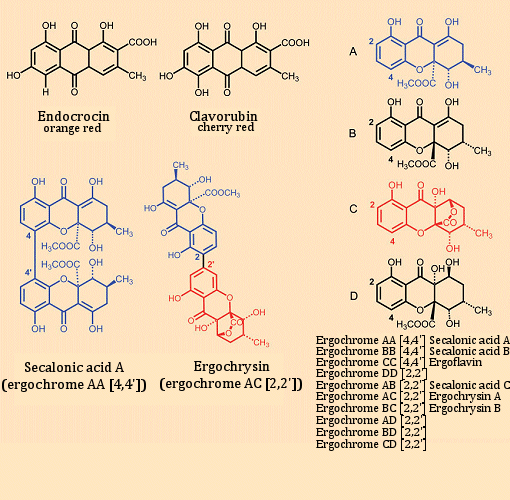
Figure 10. Pigments in ergot.
As intermediates, the building blocks A–D (see Fig. 10) are first synthesized, and these are then coupled oxidatively through their 2- or 4-positions to produce dimers. Secalonic acid A and ergochrysine are illustrative of the fundamental construction of the dimers. Altogether, ten different yellow pigments have been isolated, where secalonic acid A is the most abundant.
Most surprisingly, secalonic acid A is also synthesized by the maize fungus Penicillium oxalicum, albeit as the mirror-image isomer (enantiomer) of the ergot-derived compound [32]. The fact that here both enantiomers are found in nature for a compound with six stereogenic carbon atoms would appear to be unprecedented.
The quantities of red anthraquinone pigments (50 mg/kg) and xanthone pigments (5 g/kg) in ergot substantially surpass the corresponding alkaloid content, which is only ca. 2 g/kg. This is especially worth emphasizing since the pigments are actually more toxic than the alkaloids. For example, the pigment secalonic acid A has an LD50 value of less than 50 mg/kg [33], whereas that of the principal alkaloid, ergotamine, is “only” 62 mg/kg. One must therefore assume that the physiological effects of ergot long ascribed to alkaloids are significantly co-determined by the pigments [34].
St. Anthony’s Fire – A Disease Long “Extinct”?
The notion of a possible health threat still today from an ergot infestation of grain—such an idea has virtually disappeared from human consciousness. Ergotism or St. Anthony’s fire is now almost completely unheard of, or at most taken to be a pestilence relegated exclusively to medieval times. That all changed, however, with the European rye crop of 2003, when an ergot problem suddenly reappeared! What on earth happened?
Typical Precautionary Steps for Ergot Control
In order to protect the consumer, a maximum permissible ergot content has been established by law; in Germany, for grain intended for flour production, the ergot limit is 500 mg/kg of grain (0.05 %); for animal feed purposes, 1000 mg/kg (0.1 %). The analogous limit in the United States is 0.3 % by weight. Since the mean alkaloid content in central European ergot is ca. 0.2 %, this corresponds to a maximal alkaloid content in consumer products of roughly 1000 μg/kg for bread, and 2000 μg/kg for fodder. With respect to overall grain product generation, from seed grower or merchant through farmer, miller, and baker, these limits constitute ambitious goals, attainable in every annual harvest only with considerable effort.
Unfortunately, despite many serious endeavors, no one has yet succeeded in cultivating an ergot-resistant rye. Given that use of fungicides has also been shown to be impractical, each farmer is left with the responsibility of applying his own precautionary measures, appropriate to the life-cycle of the fungus [10]:
- In regions subject to serious ergot infestation in the previous year, alternative grains, such as barley or oats, are planted in affected fields.
- The seed grain employed must be carefully cleaned, and thus freed of ergot.
- Dense seeding leads to a consistent stand of rye, one that finishes blooming evenly and rapidly, thereby suppressing primary and secondary fungal infections.
- Since modern, highly productive hybrid varieties tend to produce little pollen, the actual pollination period is controlled and curtailed by mixing in rye varieties with a high pollination capacity.
- Wild grasses can also serve as host plants for Claviceps purpurea, so strips along the edges of fields—which would normally be left untended—are increasingly being mowed prior to blooming so that infected grasses will not spread the fungus into productive areas.
- Field perimeters with elevated ergot infestations are either harvested separately, or not harvested at all.
- Following the harvest, fields and their surrounding margins are dug up so deeply that ergot residues which may have fallen off are worked into the earth to such a depth that penetration of fruiting bodies to the surface through growth in the subsequent spring is at least inhibited.
In modern large-scale milling facilities, the quality of all newly harvested grain is determined before it is accepted. Ergot content plays an important role in this evaluation. Should this be found to exceed 0.05 %, the farmer is obliged to accept a discounted price. Ergot levels can vary drastically as a function of field location, grain variety, and crop year. For example, in 1985 at various locations in the German state of Hesse, values were reported over the wide range 0.4–1.7 % [36]. With amounts this high, mills must undertake especially thorough (and costly!) cleansing of the harvest. Pebbles, plant and insect residues, seeds from other plants, ergot, bits of iron, twigs, etc., must all be removed in a multistep process that precedes actual milling.
Separation is achieved stepwise, first with so-called “graders” as a function of particle size and form, and then in “stone sorters” based on typical specific weights for stones and metallic particles. In a modern industrial milling operation, every kernel of grain—literally every one!—is also examined with an optical sorting device using laser beams of various wavelengths, with careful evaluation. In the case of an observed color deviation, the kernel in question is separated from the normal grain stream with the aid of a 4 atm blast of air (see Fig. 11) [37]. The throughput achievable with such a system is breathtaking: more than 30,000 kernels of grain can be examined optically and then evaluated in the course of a single second!

Figure 11. Rye containing darker grains of ergot.
A combined cleansing operation of this sort can guarantee, at least in a “normal” harvest year, strict adherence to the established maximum ergot values.
So What Happened During the Annual Harvest for 2003?
In our consideration we have so far neglected to mention the most important factor in an ergot outbreak: the weather, which in 2003 failed dramatically to cooperate. A very dry, hot European summer caused the ergot kernels to remain small—roughly the same size as grains of rye, instead of being about twice the size, as is normally the case. As a result, separation based on kernel size was impossible. Moreover, the hot summer meant there was very little development of dark pigmentation, leading to insufficient distinction for sharp triggering of optical sorters. The bottom line: at least some “cleansed” European grain nevertheless ended up seriously polluted with ergot.
Certain rye samples collected in Germany in 2003 proved to have a total alkaloid level of 818 μg/kg, whereas in the subsequent year the corresponding value was only 260 μg/kg [38]. According to measurements taken by the Bundesanstalt für Getreide, Kartoffel- und Fettforschung (Federal Institute for Grain, Potato, and Fats Research), some rye flours intended for bread purposes in that same year revealed alkaloid contents of 2000–3000 μg/kg—in one case even as high as 7000 μg/kg [39]. These are very worrisome values, far exceeding the acceptable limit of 1000 μg/kg!
A Latent, Always Present Risk of Ergot
Even though the measurements above refer only to extreme outliers, they nevertheless warn us of the latent, always present risk posed by ergot. For the consumer, this raises a question regarding the utter harmlessness, from a health standpoint, of our bread. In the course of preparing dough, followed by baking it, the total alkaloid content actually sinks to about half its original value. Furthermore, the residual danger also decreases somewhat further, since the alkaloids themselves are subjected simultaneously to partial rearrangement into their pharmacologically inactive inine-forms [36].
There remains one possible potential source of hazard for the consumer who cherishes “organic” grain marketed directly by the farmer, or perhaps acquired from a small milling operation (e.g., for homemade bread), or simply self-processed by crushing or grinding, as for use in müsli (a “natural” cereal). One should also be aware of the fact that certain systems of organic farming forbid the planting of hybrid rye, which may increase the risk of ergot infestation. Even “organic” rye is generally further refined in the course of high-tech milling, however [40]. But a deliberate avoidance of all modern refinement methods can have tragic consequences. This is illustrated by the following case, drawn from clinical records [41]. It in no sense represents an isolated instance. Indeed, the number of unreported cases of suffering from ingestion of ergot alkaloids/pigments is likely to be quite large given the rather vague and diverse spectrum of associated symptoms.
In the 1980s, a thirteen-year-old female patient complained of headaches, ones that could not be associated with any specific location, as well as of “eye strain”, and of unusual spotting. Following further sudden onset of visual disorder in the form of seeing double, a series of examinations was carried out by ophthalmologists, internists, and neurologists in the course of a stay as a hospital inpatient. These led to no findings that seemed to deviate from the norm, however. The visual disorders gradually subsided over the next few days of continued hospitalization. Eventually, in the absence of any specific diagnosis, she was released and sent home. Given the way the visual symptoms had faded away, her parents attempted to adjust her diet at home so as to conform to that provided by the hospital. It turned out that in the hospital she had been required only to forego her usual breakfast of müsli. Investigation of a sample of the grain (rye) present in the family’s own müsli showed an ergot concentration of 12 %! When these breakfasts were discontinued, all the symptoms were gone.
Ergot as a Remedy
In 1770, D. T. A. Schleger described certain effects of ergot as follows [8]:
“On a fresh laceration of the finger, it quickly stanched the flow of blood and coagulated it, causing only a bit of a burning sensation and subsequently a little numbing in the wound and in the finger, but no particular inflammation was induced.”
Apart from the vasoconstrictive and styptic activity of the ergot alkaloids, their promotion of uterus contractions is also noteworthy. In 1582, Adam Lonitzer in his herbal remedy book recommended ergot (Secale cornutum) as a medicinal agent for obstetrics; Indeed, midwives had long employed it as an ecbolic or uterotonic substance [42]. Ergot was introduced into scientific medicine in 1807 by John Stearns. Based on his notes regarding properties, dosage, and side effects, ground ergot was utilized as an ecbolic “birth powder” (pulvis ad partum), although its use remained controversial over succeeding decades since this was followed unusually often by stillbirth. Pulvis ad partum thus also came to be associated with the epithet “pulvis ad mortem” (death powder) [43].
Despite such misgivings, ergot was added in 1926 to the sixth edition of the German Pharmacopoeia or “Arzneibuch” (DAB6) under the heading “Secale cornutum” [44]. It is today a drug of little commercial significance, however, especially since the alkaloid content in ergot varies to an extraordinary extent [20], making reliable specification of a dosage virtually impossible. Around 1900, the so-called “cockscomb test” was commonly employed for assessing the potency of an ergot preparation. In this procedure, a rooster was injected intramuscularly with a defined quantity of a particular ergot extract. (A substance with a known alkaloid content was required for comparison.) The assessed variable was the extent to which circulation was impaired in the bird, as judged by the appearance of its cockscomb. Incidentally, a given rooster could not be subjected to the procedure again until at least two weeks had elapsed [7].
Apart from the substantial problems associated with precise dosage, ergot’s serious side effects have caused it to completely disappear from conventional medicine. It did experience a certain renaissance after Stoll succeeded in 1918 in isolating from it the pure substance ergotamine [20]. Today, however, only the pure components are employed, along with associated semi-synthetic derivatives. Chief applications for these are in the field of obstetrics, along with migraine (see below) and Parkinson’s therapy.
Obstetric Applications
The effect of ergot on contractions during labor is quite unique: after administration of an aqueous extract of ergot, first weak contractions begin within a few minutes, and these lead after ca. one hour to contractions that are regular and powerful. It was formerly the case that no other drug was known to prompt such labor activity. The specific ergot alkaloid responsible for this effect, ergometrin, was isolated in 1935 [45]. Ergometrin and its semi-synthetic derivative methylergometrin still see some application in this context, especially due to their hemostatic characteristics. Methylergometrin is also approved for use with the bleeding that may follow a miscarriage or abortion, as well as with possible increased hemorrhaging after delivery, and for support in postpartum uterine contraction, but here only for mothers not engaging in breast-feeding [46].
As beneficial as this hemostatic activity can be, the associated vasoconstriction often leads to undesirable effects, since cardiovascular constriction of as much as 20 % can occur. There have been numerous reports of this leading in turn to fatal heart attacks [46].
Migraines
For a long time, ergotamine and its semi-synthetic hydrogenation derivative dihydroergotamine were the only therapeutic agents available for migraine. Migraine is a condition whose origin has still not been totally clarified even today, although it is certain that its extreme headaches are a consequence of dilation of blood vessels in the brain.
Ergotamine, and the more readily tolerated dihydroergotamine, cause the extreme headaches to relent, typically within an hour, as a direct result of their vasoconstrictive activity [47]. One major risk associated with their use relates to potential overdosing, however. Should a patient take more than the prescribed quantity of these agents, an unfortunate side effect is likely to arise—namely a headache [47]! The patient might then be tempted to take even more ergotamine, resulting in a vicious cycle.
Migraine prophylaxis is another area of application for ergot alkaloids. Methysergide was introduced in 1959 as a preventative drug, and early clinical studies showed outstanding results [48]. But only five years later it became clear that too many patients who had taken the drug on a regular basis developed severe retroperitoneal fibrosis (Morbus Ormund). Another serious side effect can be left-sided cardiac valve dysfunction. For these reasons, methysergide is seldom employed today. Currently, drugs from the triptane category are the preferred choice for migraine therapy.
Isolation of Ergot Alkaloids
Until a few decades ago, the raw materials necessary for production of ergot-derived pharmaceuticals were obtained from agricultural sources. Thus, specific rye fields were deliberately infected with Claviceps purpurea. The resulting yield of ergot was on the order of 200–500 kg/ha (ca. 180–450 lb/acre) [11]. Increasing demand for ergot alkaloids led, however, to alternative, strictly industrial production techniques [49]. Thus, culturing the ergot sclerotia on agar leads to white mycels that can readily be transferred to a liquid culture medium for further cultivation in aerated fermenters. Over the course of two to three weeks, the water-soluble alkaloids, such as ergometrin, are released into the culture medium, whereas the peptide alkaloids can be extracted directly from the mycels (see Fig. 7). The yield of alkaloids is substantial: on the order of 2 g/L of nutrient solution [49].
Alkaloid derivatives of industrial origin are today based exclusively on ergot alkaloids generated by biotechnological means.
References
[18] “St. Anthony’s Gift”, Eur. Rev. 2003, 11, 27.
[19] H. Remane, ChemKon 2009, 16, 164. DOI: 10.1002/ckon.200990024
[20] A. Stoll, Helv. Chim. Acta 1945, 28, 1283. DOI: 10.1002/hlca.6602801182
[21] D. Gröger, Fortschr. Chem. Forsch. 1966, 6, 159. DOI: 10.1007/BFb0051579
[22] A. Stoll, A. Hofmann, Helv. Chim. Acta 1943, 26, 1570 (a brief historical summary). DOI: 10.1002/hlca.19430260522
[23] S. Smith, G. M. Timmis, J. Chem. Soc. 1932, 1543. DOI: 10.1039/jr9320001543
[24] W. A. Jacobs, L. C. Craig, Science 1935, 81, 256. DOI: 10.1126/science.81.2097.256-a
[25] W. A. Jacobs, L. C. Craig, J. Org. Chem. 1936, 1, 245. DOI: 10.1021/jo01232a003
[26] E. C. Kornfeld, R. B. Woodward et al., J. Am. Chem. Soc. 1954, 76, 5256. DOI: 10.1021/ja01649a100
[27] A. Hofmann et al., Experientia 1961, 17, 206. DOI: 10.1007/BF02160615
[28] I. Moldvai et al., Helv. Chim. Acta 2005, 88, 1344. DOI: 10.1002/hlca.200590108
[29] H.G. Floss, Tetrahedron 1975, 32, 873.
[30] W. Schlientz, A. Hofmann et al., Experientia 1961, 17, 108. DOI: 10.1007/BF02160811
[31] B. Franck, Angew. Chem. 1969, 81, 269. DOI: 10.1002/ange.19690810802
[32] P. S. Steyn, Tetrahedron 1970, 26, 51. DOI: 10.1016/0040-4020(70)85006-2
[33] B. Franck, Angew. Chem. 1984, 96, 462. DOI: 10.1002/ange.19840960705
[34] M. Yamazaki et al., Chem. Pharm. Bull. 1971, 19, 199. DOI: 10.1248/cpb.19.199
[35] Getreidemarktordnung Nr. 824/200 for 19 April, 2000.
[36] J. Wolff et al., Z. Ernährungswiss. 1988, 27, 1. DOI: 10.1007/BF02021291
[37] www.muehle-heiligenrode.de/mtrieur.htm.
[38] U. Lauber et al., Mycotoxin Res. 2005, 21, 258. DOI: 10.1007/BF02957588
[39] Stellungnahme des Bundesinstituts für Risikobewertung for 22 January 2004.
[40] U. Lauber et al., Mycotoxin Res. 2005, 21, 258.
[41] H. J. Pfänder et al., Deutsches Ärzteblatt 1985, 82, 2013.
[42] R. Giebelmann, Toxichem Krimtech 2002, 1, 30.
[43] Journal für Geburtshülfe, Frauenzimmer- und Kinderkrankheiten (Ed. V. Siebold), Vol. 10, Verlag Wilhelm Engelmann, Leipzig, Germany, 1837.
[44] Deutsches Arzneibuch (German Pharmacopoeia), 6th ed., Verlag Decker, Berlin, Germany, 1926.
[45] P. van Dongen, A. de Groot, Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 60, 109.
[46] W. Rath, W. Gogarten, Frauenarzt 2008, 49, 498.
[47] P. C. Tfelt-Hansen, P. J. Koehler, Cephalalgia 2008, 28, 877. DOI: 10.1111/j.1468-2982.2008.01578.x
[48] P. C. Tfelt-Hansen, P. J. Koehler, Cephalalgia 2008, 28, 1126. DOI: 10.1111/j.1468-2982.2008.01648.x
[49] R. Hänsel et al., Hagers Handbuch der pharmakologischen Praxis, Drogen A–D, Springer, Berlin, Germany, 1992.
Prof. Klaus Roth
Freie Universität Berlin, Germany.
Dr. Sabine Streller
Freie Universität Berlin, Germany.
The article has been published in German in:
and was translated by W. E. Russey.
A Chemical Examination of the Isenheim Altar: Role Played in History by “Horned Rye” Part 1
Takes a scientific look at the Isenheim Altar, which depicts the symptoms and treatment of “St. Anthony’s Fire”, the result of poisoning by ergot alkaloids
A Chemical Examination of the Isenheim Altar: Role Played in History by “Horned Rye” Part 2
Looks at what it means when a kernel of grain is infected with ergot, the life-cycle of the fungus, and how it may have contributed to the Salem witch trials
Other articles by Klaus Roth published by ChemViews magazine:
- In Espresso — A Three-Step Preparation
Klaus Roth proves that no culinary masterpiece can be achieved without a basic knowledge of chemistry
DOI: 10.1002/chemv.201000003 - In Chocolate — The Noblest Polymorphism
Klaus Roth proves only chemistry is able to produce such a celestial pleasure
DOI: 10.1002/chemv.201000021 - In Sparkling Wine, Champagne & Co
Klaus Roth shows that only chemistry can be this tingling
DOI: 10.1002/chemv.201000047 - In Chemistry of a Hangover — Alcohol and its Consequences
Klaus Roth asks how can a tiny molecule like ethanol be at the root of so much human misery?
DOI: 10.1002/chemv.201000074 - In The Chemist’s Fear of the Fugu
Klaus Roth shows that the chemist’s fear of the fugu or pufferfish extends as far as the distinctive and intriguing poison it carries
DOI: 10.1002/chemv.201000104 - In Chemistry of a Christmas Candle
Klaus Roth explains that when we light a candle, the chemistry we are pursuing is not only especially beautiful, but also especially complex
DOI: 10.1002/chemv.201000133 - In Pesto — Mediterranean Biochemistry
Klaus Roth uncovers the nature of this culinary-chemical marvel, and thereby comes to enjoy it all the more
DOI: 10.1002/chemv.201200001 - In Boiled Eggs: Soft and Hard
Klaus Roth examines an egg on its journey from hen to table to ensure the perfect breakfast egg
DOI: 10.1002/chemv.201200018 - Chemical Secrets of the Violin Virtuosi
What made Stradivari’s violins so special? Klaus Roth looks at the important role of chemistry in Stradivari’s workshop and instruments
DOI: 10.1002/chemv.201200076 - Chemical Production in Compliance with Torah and the Koran
The unusual interface between chemistry and religion, drawing upon examples from Islamic and Judaic law
DOI: 10.1002/chemv.201200088 - Video Interview with Klaus Roth
See all articles published by Klaus Roth in ChemViews Magazine



